Biological amniotic membrane with complete cell structure and controllable degradation and preparation method thereof
An amniotic membrane and biological technology, applied in the field of biomedicine, can solve the problems of large cell proliferation and anti-inflammatory loss, easy destruction of amniotic membrane collagen and fibrous structure, reduced repair and anti-inflammatory effects, etc., and achieve good clinical treatment, cell and collagen structure. full effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
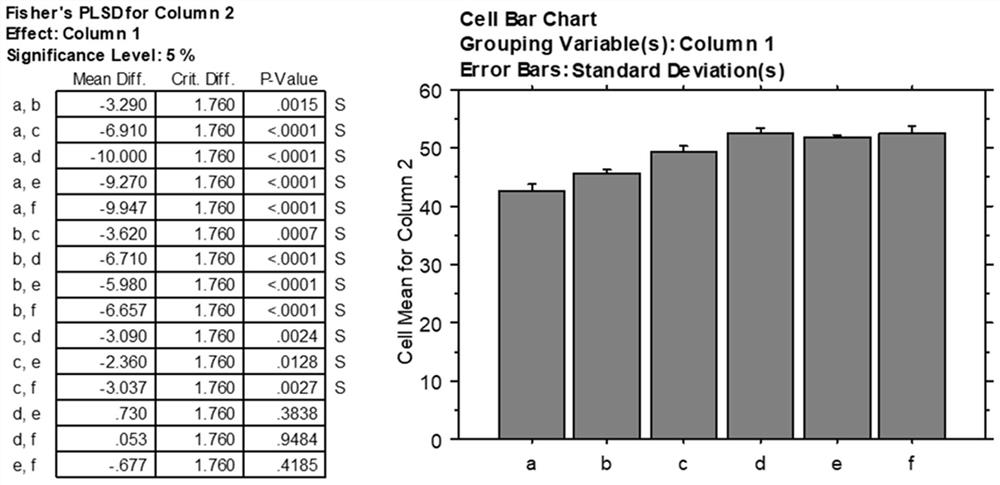
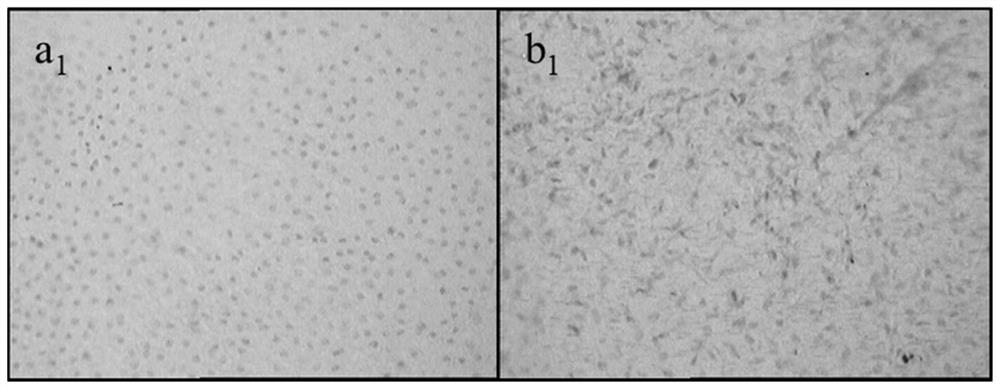
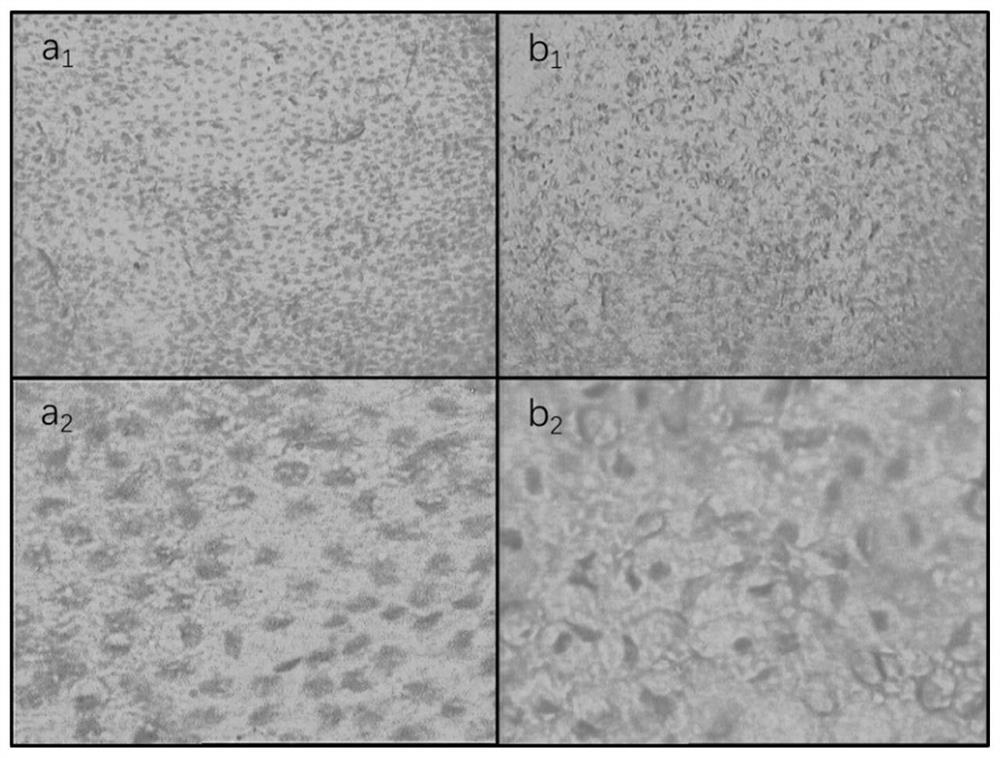
[0040] Embodiment 1 crosslinking agent is 0.05%, 0.1%, 0.25%, 0.5%, 1%, 2% glutaraldehyde solution
[0041] This embodiment provides the biological amniotic membrane obtained when the cross-linking agent concentration is 0.05%, 0.1%, 0.25%, 0.5%, 1%, and 2%; the specific steps are as follows:
[0042] (1) Spread the fresh amnion, and use a smooth sheet stainless steel scraper to manually scrape it against the growth direction of the chorion on the surface of the amnion to obtain amnion without chorion;
[0043] (2) Soak the amnion after scraping off the chorion in 3% hydrogen peroxide for 2 hours;
[0044] (3) Place the amniotic membrane soaked in hydrogen oxide in purified water for three consecutive washes, and then place it in 75% ethanol for 2 hours;
[0045] (4) Place the amniotic membrane treated with hydrogen peroxide and ethanol in purified water for three consecutive washes, and place them in cross-linking agent concentrations of 0.05%, 0.1%, 0.25%, 0.5%, 1%, and 2%,...
Embodiment 2
[0051] Embodiment 2 cross-linking agent is 0.1% glutaraldehyde solution
[0052] This embodiment provides the bio-amniotic membrane prepared when the cross-linking agent is 0.1% glutaraldehyde solution, and the specific steps are as follows:
[0053] (1) Spread the fresh amnion, and manually scrape it against the growth direction of the chorion on the surface of the amnion with a smooth sheet-shaped stainless steel scraper to obtain chorion-free amnion;
[0054] (2) Soak the amnion after scraping off the chorion in 3% hydrogen peroxide for 2 hours;
[0055] (3) Place the amniotic membrane soaked in hydrogen oxide in purified water for three consecutive washes, and then place it in 75% ethanol for 2 hours;
[0056] (4) Place the amniotic membrane treated with hydrogen peroxide and ethanol in purified water for three consecutive washes, then place it in a 0.1% glutaraldehyde solution, place it in a shaker, and set the shaker speed to 100r / min, the time is set to 1h, and the c...
Embodiment 3
[0059] Embodiment 3 cross-linking agent is 0.2% genipin solution
[0060] This embodiment adopts the same preparation method as in Example 2, the difference is that the cross-linking agent used is 0.2% genipin solution, the shaker speed is set to 50r / min, and the time is set to 30min for cross-linking treatment; Specific steps are as follows:
[0061] (1) Spread the fresh amnion, and use a smooth sheet stainless steel scraper to manually scrape it against the growth direction of the chorion on the surface of the amnion to obtain amnion without chorion;
[0062] (2) Soak the amnion after scraping off the chorion in 3% hydrogen peroxide for 2 hours;
[0063] (3) Place the amniotic membrane soaked in hydrogen oxide in purified water for three consecutive washes, and then place it in 75% ethanol for 2 hours;
[0064] (4) Place the amniotic membrane treated with hydrogen peroxide and ethanol in purified water for three consecutive washes, then place it in a genipin solution with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com