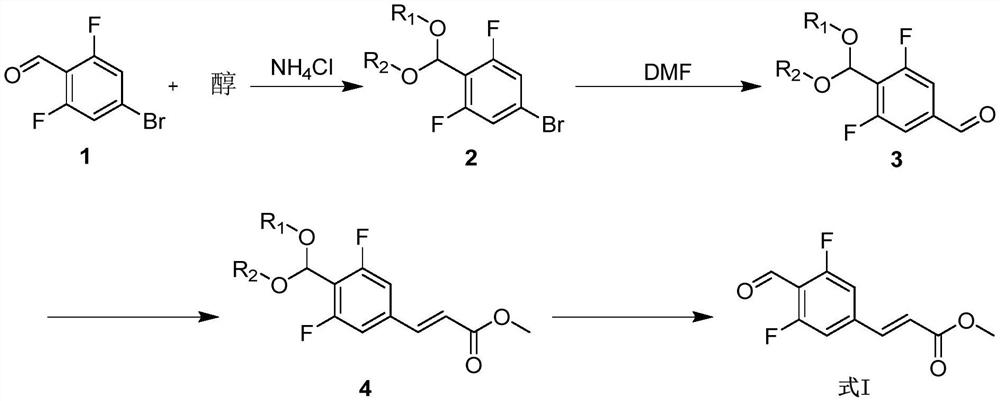
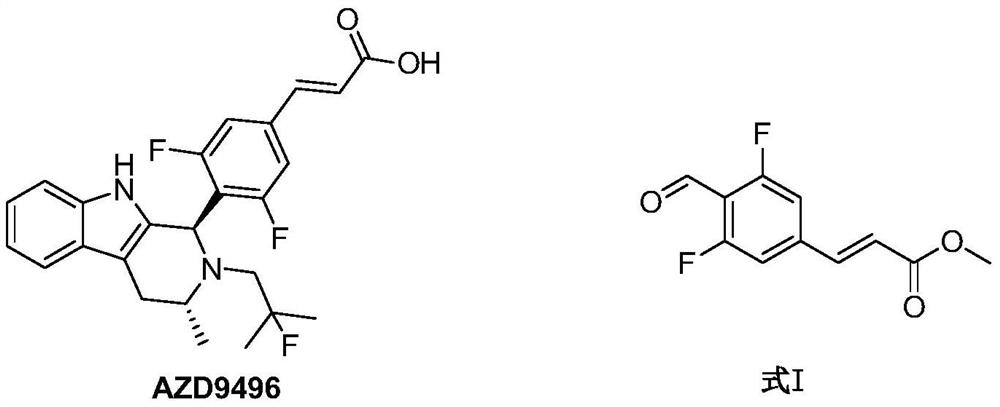
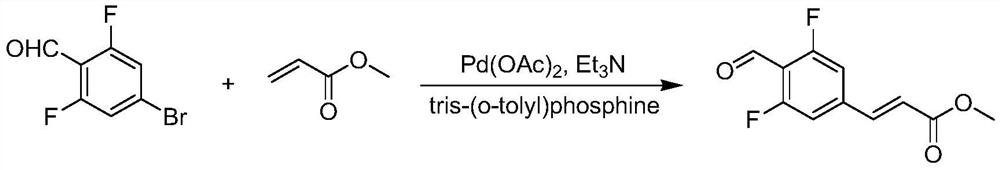
Synthesis method of (E)-methyl ester 3-(3,5-difluoro-4-formyl phenyl)acrylic acid
The technology of a formyl phenyl group and a synthesis method, which is applied in the synthesis field of -methyl 3-acrylic acid, can solve the problems of high cost, unsuitability for mass production, unfriendly environment and the like, and achieves high safety, good application prospect, Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1, the synthesis method of (E)-methyl ester 3-(3,5-difluoro-4-formylphenyl)acrylic acid of the present invention (1) Preparation of compound 2a-c
[0049] Preparation of compound 2a:
[0050]
[0051]To a suspension of compound 1 (180 g, 0.814 mol) in methanol (900 mL) were added trimethyl orthoformate (112.4 g, 1.059 mol) and ammonium chloride (2.2 g, 0.041 mol). React at 45-55°C for 20 hours. Concentrate under reduced pressure, add MTBE (600 mL), wash with saturated sodium bicarbonate (150 mL), wash with saturated sodium chloride (150 mL), and concentrate under reduced pressure to obtain a crude product. The crude product was purified by distillation to obtain 201 g of light yellow liquid with a yield of 92.4%.
[0052] Preparation of compound 2b:
[0053]
[0054] To a suspension of compound 1 (100 g, 0.452 mol) in ethanol (500 mL) was added triethyl orthoformate (95 g, 0.641 mol) and ammonium chloride (1.3 g, 0.024 mol). React at 45-55°C for 20 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com