A class of singlet oxygen-type photosensitizer materials with aggregation-induced luminescence properties and their preparation methods and applications
A technology of aggregation-induced luminescence and singlet oxygen, which is applied in the field of biomedical materials, can solve problems such as unfavorable fluorescence-mediated photodynamic therapy, and achieve the effects of high-efficiency cure, high-specific targeting, and good lethality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Preparation of a singlet oxygen-type photosensitizer material with aggregation-induced luminescence properties (singlet oxygen photosensitizer, marked as PTI in Example 1)
[0057]
[0058] The synthetic route is as follows:
[0059]
[0060] a) Synthesis of compound 10-phenyl-10H-phenothiazine (1): add phenothiazine (6g, 30mmol), copper powder (1.9g, 30mmol), potassium carbonate (8.3g, 60mmol) to a 100ml two-necked flask ) and 18-crown ether-6 (3g, 60mmol), nitrogen-pumping-nitrogen cycle 3 times (10 minutes each time), then add iodobenzene 3.93ml and ultra-dry N,N-dimethylformamide 60mL , heated to 180°C for 12 hours, the reaction was over, extracted with dichloromethane / water, dried over anhydrous magnesium sulfate for 2 hours, separated and purified by column layer analysis to obtain colorless crystals (4.2g) with a yield of 60%. 1 H NMR (500MHz, CDCl 3 ), 7.60(t, 2H), 7.46(t, 1H), 7.38(d, 2H), 7.01(d, 2H), 6.82(m, 4H), 6.20(d, 2H).
[0061] b) Sy...
Embodiment 2
[0064] Example 2 Preparation of a singlet oxygen-type photosensitizer material with aggregation-induced luminescence properties (singlet oxygen photosensitizer, marked as PI in Example 2)
[0065]
[0066] The synthetic route is as follows:
[0067]
[0068] a) The synthesis procedure of the intermediate 10-phenyl-10H-phenothiazine (1) is the same as that of Example 1.
[0069] b) Synthesis of compound 10-phenyl-10H-phenothiazine-3-aldehyde (2): add ultra-dry N,N-dimethylformamide solvent ( 2.8 mL) and 1,2-dichloroethane (5 mL), and after stirring for ten minutes, phosphorus oxychloride (2.1 mL) was added dropwise to the mixture. Then, 10-phenyl-10H-phenothiazine (1 g, 3.6 mmol) dissolved in 5 mL of 1,2-dichloroethane solution was added dropwise within 30 min, heated to 90° C., and the reaction was continued for 12 h. After stopping the reaction and cooling to room temperature, the mixture was poured into 100 mL of ice-water and washed with saturated NaHCO 3 The solut...
Embodiment 3
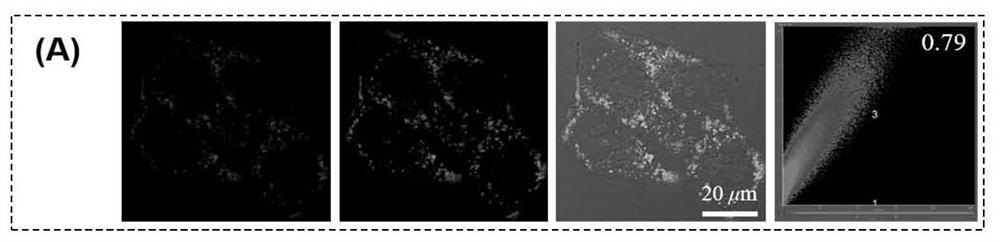
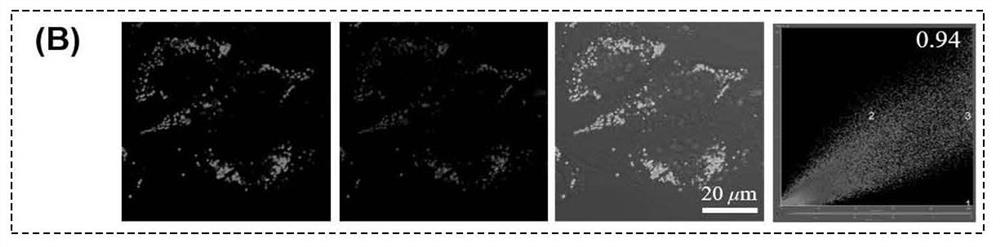
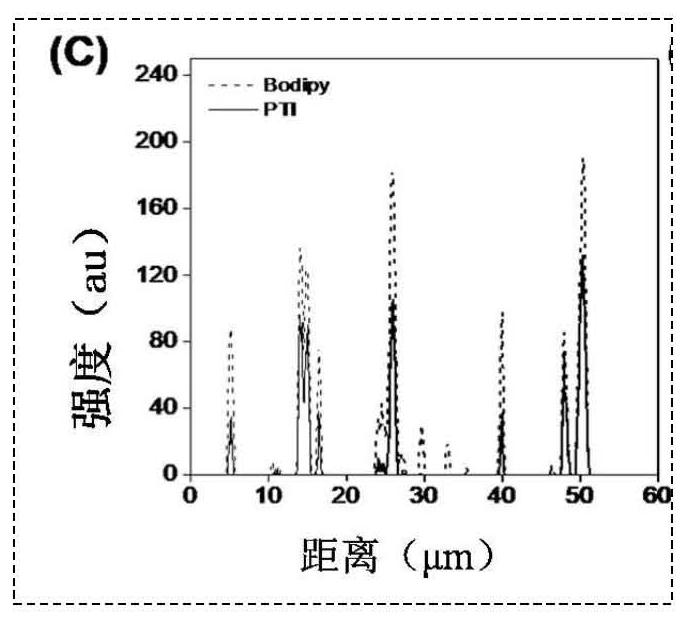
[0071] Example 3 Based on the specific targeting of phenothiazine photosensitizers (PI and PTI) to lipid droplets After culturing MCF-7 cells for 24 hours, the cells were first incubated with oleic acid for 1 hour, followed by the addition of 10 μM PI and PTI, respectively The cells were incubated for 6 h, then the original medium was removed and washed twice with PBS, and fresh medium containing the lipid droplet dye Bodipy (final concentration 2 μM) was added to continue incubation for 20-30 min, washed and replaced with fresh medium for confocal microscopy imaging characterization. Figure 1a and Figure 1b After incubation with cells for 6 hours for the PTI prepared in Example 1 and the PI prepared in Example 2, respectively, the lipid droplet dyeing effect of the cells was performed with the lipid droplet dye BODIPY. Figure 1c and Figure 1d Co-staining index intensity map of lipid droplet co-staining with lipid droplet dye BODIPY after incubation with cells for PTI prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com