A fluorescent probe molecule for detecting azoreductase based on coumarin derivatives and its preparation method and application
A technology of coumarin derivatives and fluorescent probes, which is applied in the field of fluorescent probe molecules and their preparation, can solve problems such as the rare research work on azoreductase probes, achieve good stability of physical and chemical properties, and yield High rate, clear structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
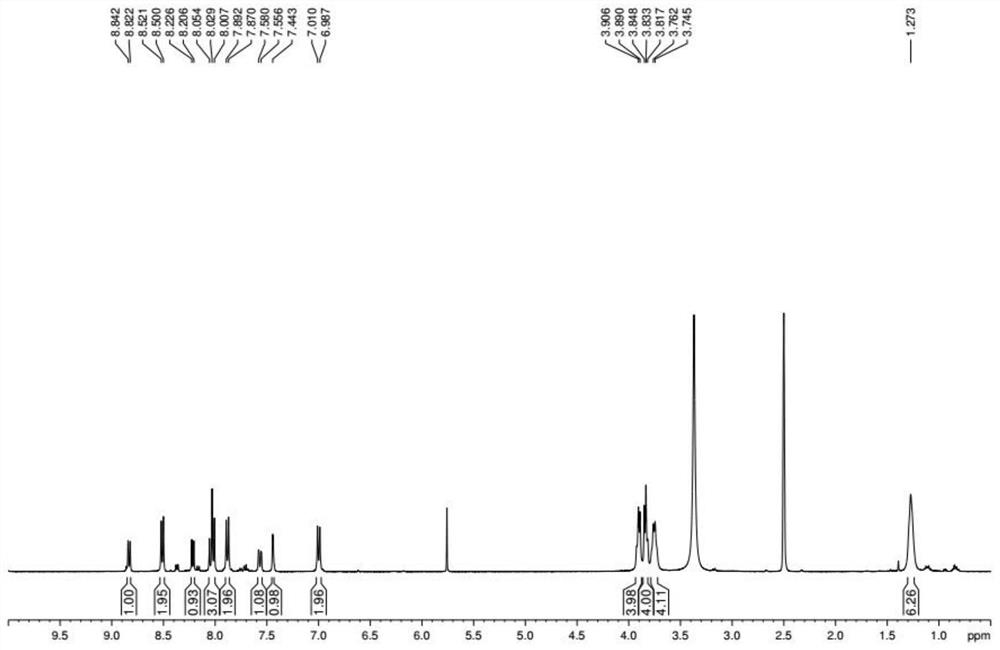
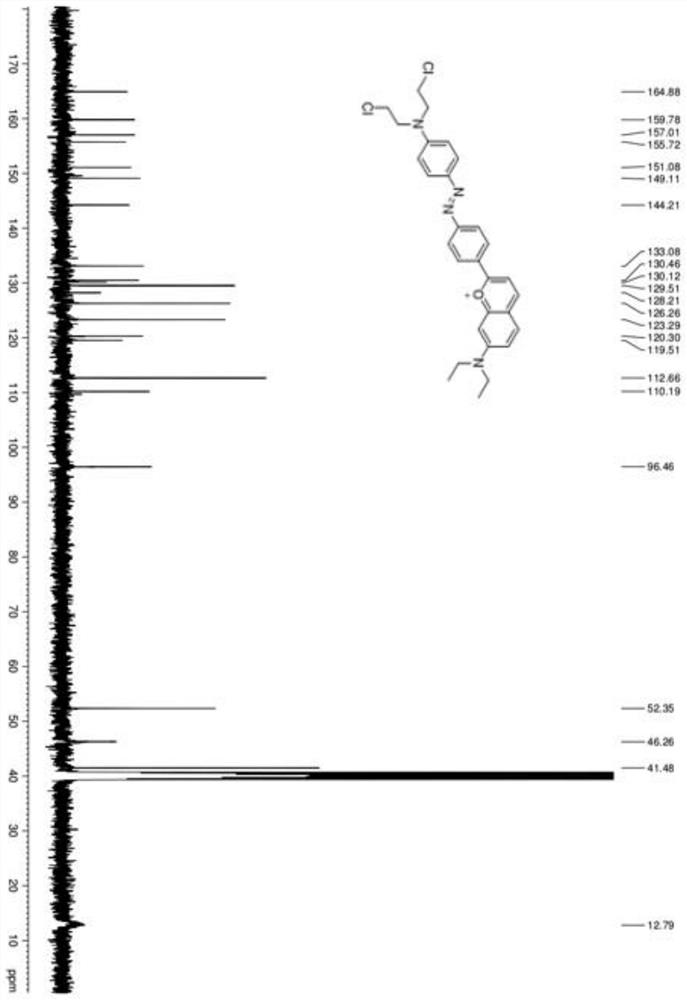
Image
Examples
preparation example Construction
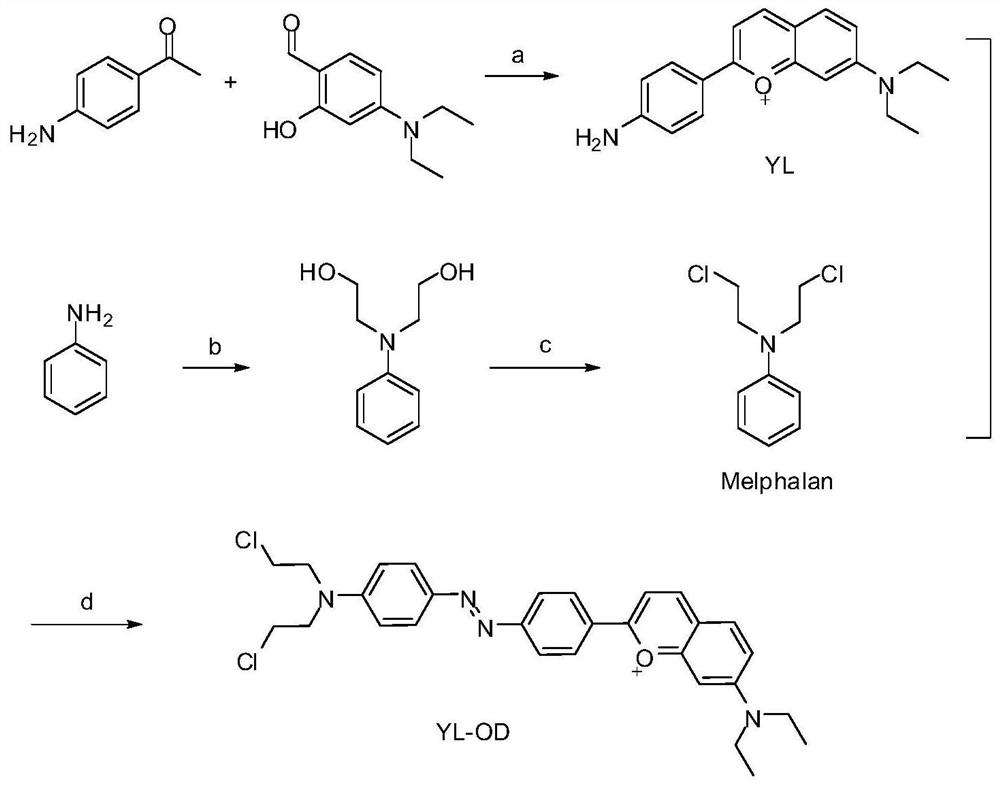
[0048] The present invention provides the preparation method of the fluorescent probe molecule based on coumarin derivatives to detect azoreductase, see figure 1 , including the following steps:
[0049] 1) under the action of sulfuric acid, 4-aminoacetophenone and 4-(diethylamino) salicylaldehyde are mixed for condensation reaction to obtain a first reaction solution;
[0050] 2) Mixing the first reaction solution described in step 1) and perchloric acid to undergo a ring-forming reaction to obtain a compound YL having a structural formula as shown in Formula II;
[0051]
[0052] 3) under the action of hydrochloric acid, compound YL and NaNO described in step 2) 2 Mixing occurs a diazotization reaction to obtain a diazonium salt and obtain a compound shown in formula III;
[0053]
[0054] 4) Under the action of aqueous sodium hydroxide solution, aniline and 2-bromoethanol are mixed for a substitution reaction to obtain N-phenyldiethanolamine;
[0055] 5) N-phenyldi...
Embodiment 1
[0084] The specific synthetic route of fluorescent probe molecules based on coumarin derivative azoreductase detection is shown in Figure 1.
[0085] (1) Add 4-aminoacetophenone (13.5g, 0.1mol) and 4-(diethylamino) salicylaldehyde (19.3g, 0.1mol) in the round bottom flask, add 50mL of analytically pure sulfuric acid at 400-450rpm The mixture was stirred at a rotating speed, and the temperature was raised to 90° C. for 6 h. After the reaction was completed, the reaction solution was poured into ice water, and 0.3 times the volume of the reaction solution was added with 70% perchloric acid solution. Stir for 15 minutes to precipitate precipitates, filter with suction, wash the filter cake with water, and dry to obtain a purple-black solid, which is purified by silica gel column chromatography (200 mesh silica gel) and eluent (methanol:dichloromethane=1:100, volume ratio) Elution gave compound YL (14.5 g, yield 49.5%).
[0086] (2) Add 2-bromoethanol (2.48g, 20mmol) and aniline...
Embodiment 2
[0096] Detection of Sn by Fluorescent Probe Molecule Based on Coumarin Derivative Azoreductase 2+ Selectivity of fluorescence detection
[0097] A fluorescence spectrophotometer (model F97XP of Shanghai Lenslight Technology Co., Ltd.) was used to measure the concentration of the probe on Sn 2+ With and without Sn 2+ of the fluorescence emission spectrum. see results Figure 5 . Depend on Figure 5 It can be seen that without adding Sn 2+ When , the probe has no fluorescence emission at 620nm; while at Sn 2+ In the presence of the probe, the fluorescence intensity of the probe was enhanced by nearly 450 times, indicating that the probe has a strong effect on the Sn 2+ There is a better response.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com