Application of bis(fluorosulfonyl)imide as catalyst
The technology of bisfluorosulfonimide and catalyst is applied in the application field of bisfluorosulfonimide as a catalyst, which can solve the problems of narrow application field of bisfluorosulfonimide, and achieves strong substrate adaptability and atom economy. High performance and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0269]
[0270] Add 10 g of potassium bisfluorosulfonimide (45.6 mmol) and 10 g of 98% concentrated sulfuric acid (100 mmol) into the autoclave, feed 60 g of sulfur dioxide at -70°C, slowly rise to room temperature, react for half an hour, and a white solid appears Generate, then slowly release sulfur dioxide gas, add 50ml of dichloromethane, filter, the solid is washed with 50ml of dichloromethane, the filtrate is spin-dried, and distilled under reduced pressure to collect 85 ° C / 20mm Hg fractions to obtain 7.7 g of difluorosulfonimide ( 42.5 mmol), colorless liquid, yield 93%.
[0271] 19 F NMR (dichloromethane as solvent, fluorotrichloromethane as internal standard): +57.3.
preparation Embodiment 2
[0273]
[0274] Add 10g of sodium bisfluorosulfonimide (49.26mmol) and 10g of 98% concentrated sulfuric acid (100mmol) into the autoclave, feed 60g of sulfur dioxide at -70°C, slowly rise to room temperature, react for half an hour, and there is a white solid Generate, then slowly release sulfur dioxide gas, add 50ml of dichloromethane, filter, wash the solid with 50ml of dichloromethane, spin the filtrate, distill under reduced pressure, collect 85 ° C / 20mm Hg fractions, and obtain 8.1 g of difluorosulfonimide ( 44.75 mmol), colorless liquid, yield 91%.
[0275] 19 F NMR (dichloromethane as solvent, fluorotrichloromethane as internal standard): +57.3.
Embodiment 1
[0277]
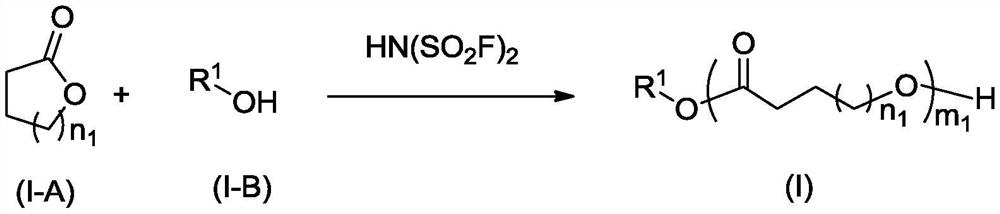
[0278] In a 25ml three-necked flask, add 1.9998g (17.54mmol, 1eq) of caprolactone, 5ml of dichloromethane, 175ul concentration of phenylpropanol dichloromethane solution (1%eq) of 1mol / L, add 35ul under nitrogen protection conditions The dichloromethane solution (0.1% eq) of bisfluorosulfonylimide acid with a concentration of 0.5mol / L was reacted overnight at room temperature. Lactone solid 1.86g (yield 93%) was detected by GPC, Mn PS =25267, PDI=1.03.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com