A kind of method of room temperature redox direct synthesis magnesium borohydride
A magnesium borohydride, direct technology, applied in the direction of borane/diborane hydride, etc., can solve the problems of high requirements for preparation conditions, high equipment requirements, high risk coefficient, etc., and achieves low price, high safety, source of rich effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
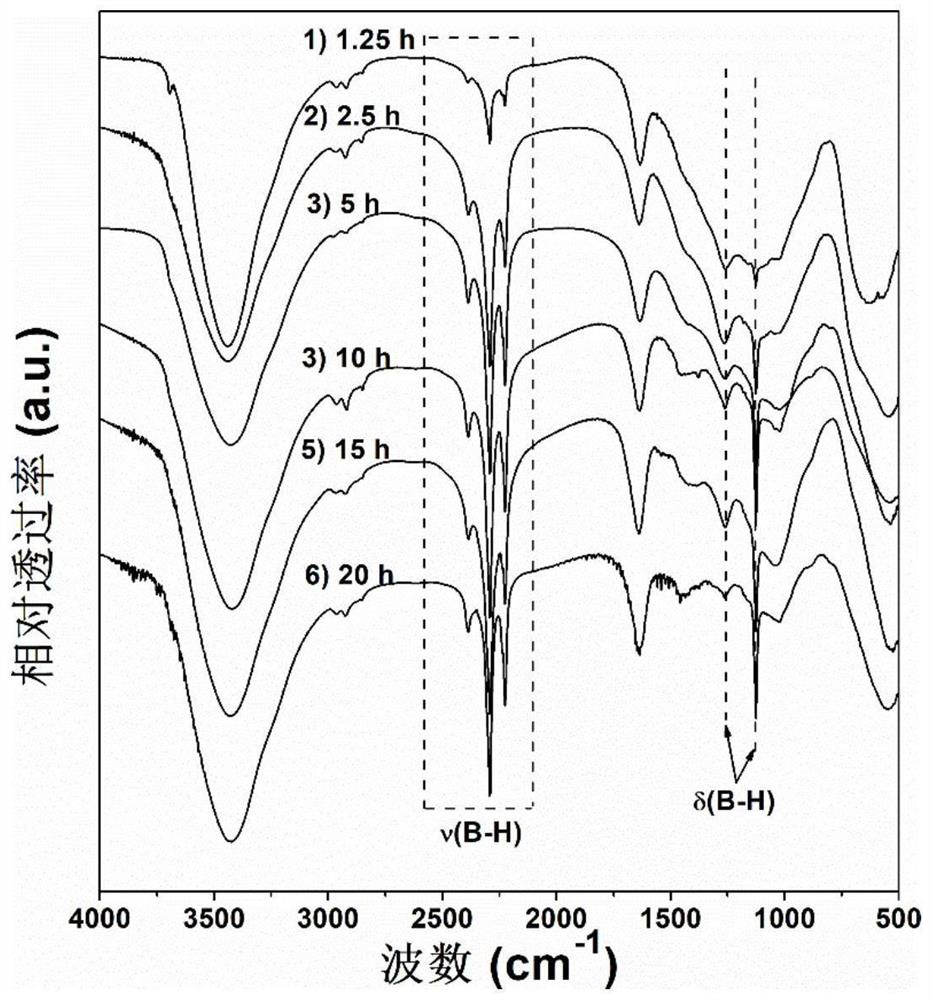
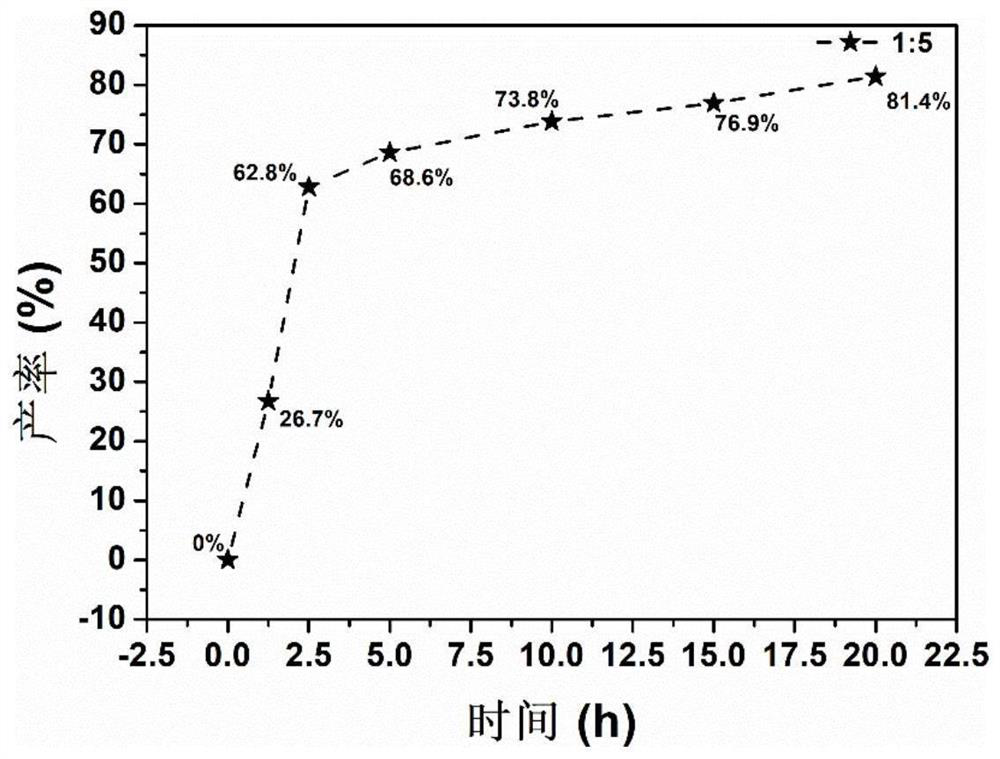
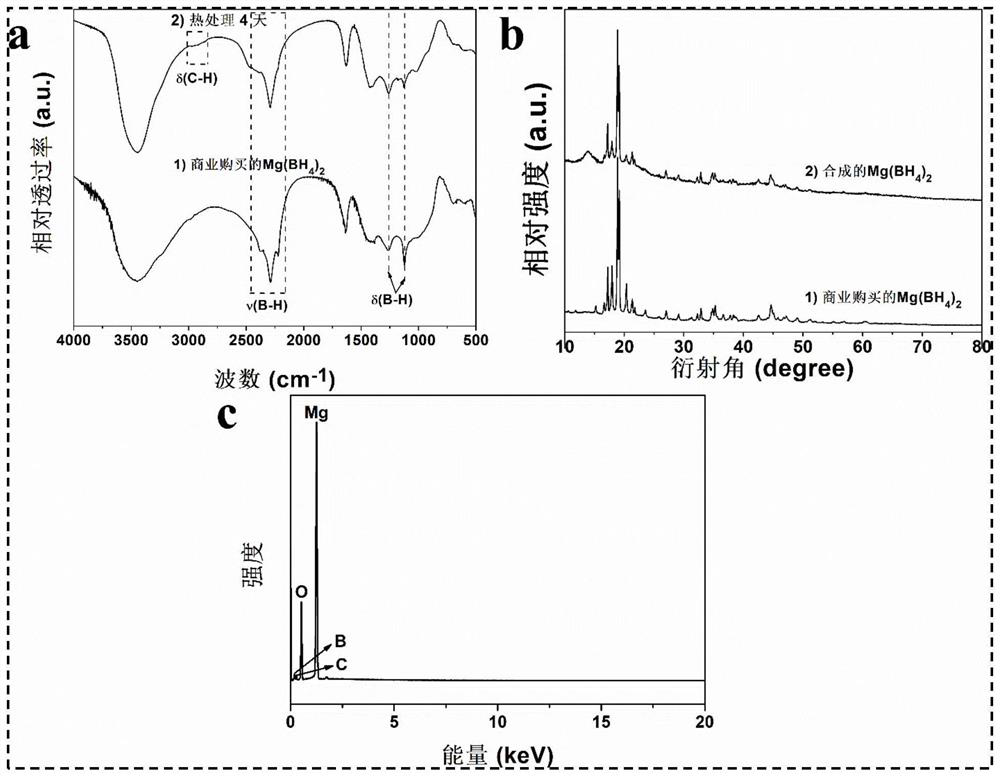
[0060] In a glove box with an argon atmosphere of 0.1MPa, boron oxide and magnesium hydride were weighed at a molar ratio of 1:5, mixed and put into a ball mill and placed in a vibration ball mill (QM-3C). The material ratio is 50:1, the ball milling speed is 1000 rpm, and the ball milling is directly carried out under the argon atmosphere for 1.25 hours to obtain a ball milling product. figure 1 The middle curve 1) is the FTIR spectrogram of the ball mill product, and the middle curve is 2150-2400cm -1 and 1100-1300cm -1 The corresponding Mg(BH 4 ) 2 The B-H bond stretching vibration (ν in the figure, the same below) and rocking vibration (δ in the figure, the same below) absorption peaks in the figure prove that magnesium borohydride has been successfully synthesized; Dissolve and filter to obtain a clear filtrate, use Schlenk technology to dry the filtrate to obtain a viscous magnesium borohydride solvent compound, then digest it with acid and configure it into a solutio...
Embodiment 2
[0062] In a glove box with an argon atmosphere of 0.1MPa, boron oxide and magnesium hydride were weighed at a molar ratio of 1:5, mixed and put into a ball mill and placed in a vibration ball mill (QM-3C). The material ratio is 50:1, the ball milling speed is 1000 rpm, and the ball milling is directly carried out under the argon atmosphere for 2.5 hours to obtain a ball milling product. figure 1 Middle curve 2) is the FTIR spectrogram of this ball mill product, in the curve 2150-2400cm -1 and 1100-1300cm -1 The corresponding Mg(BH 4 ) 2 The B-H bond stretching and rocking vibration absorption peaks in the figure prove that magnesium borohydride was successfully synthesized; the mixture was dissolved and filtered to obtain a clear filtrate using diethyl ether solvent distilled from sodium to obtain a viscous Magnesium borohydride solvent compound, digested with acid and configured into a solution, then use the inductively coupled plasma spectrometer to measure the concentrat...
Embodiment 3
[0064] In a glove box with an argon atmosphere of 0.1MPa, boron oxide and magnesium hydride were weighed at a molar ratio of 1:5, mixed and put into a ball mill and placed in a vibration ball mill (QM-3C). The material ratio is 50:1, the ball milling speed is 1000 rpm, and the ball milling is directly carried out under the argon atmosphere for 5 hours to obtain a ball milling product. figure 1 Middle curve 3) is the FTIR spectrogram of this ball mill product, in the curve 2150-2400cm -1 and 1100-1300cm -1 The corresponding Mg(BH 4 ) 2 The B-H bond stretching and rocking vibration absorption peaks in the figure prove that magnesium borohydride was successfully synthesized; the mixture was dissolved and filtered to obtain a clear filtrate using diethyl ether solvent distilled from sodium to obtain a viscous Magnesium borohydride solvent compound, digested with acid and configured into a solution, then use the inductively coupled plasma spectrometer to measure the concentratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com