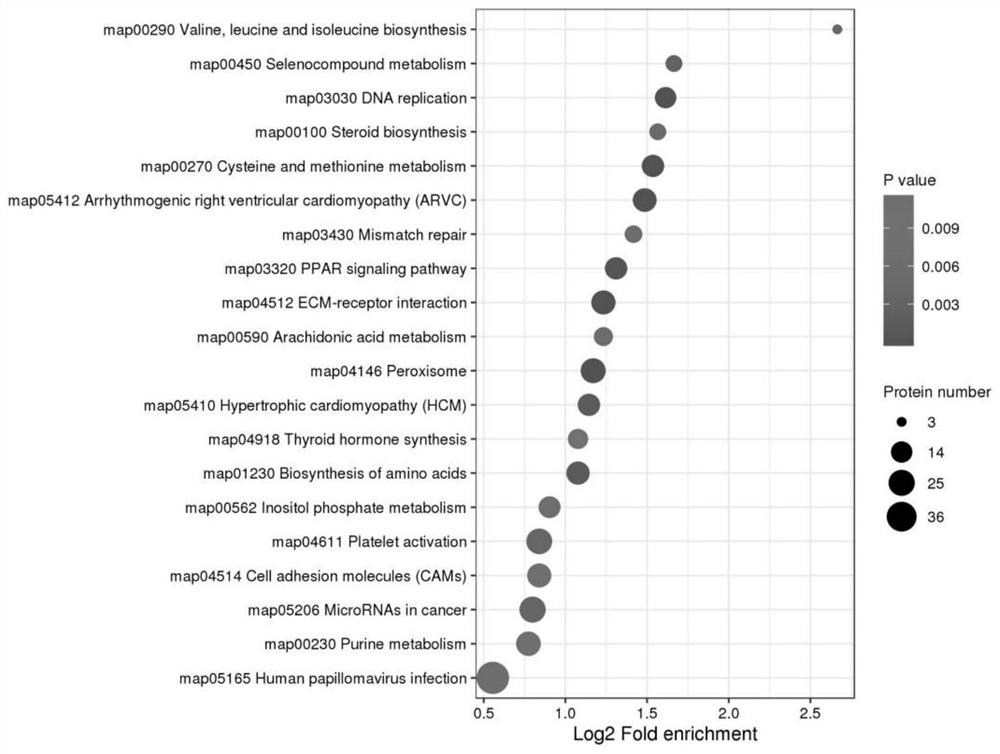
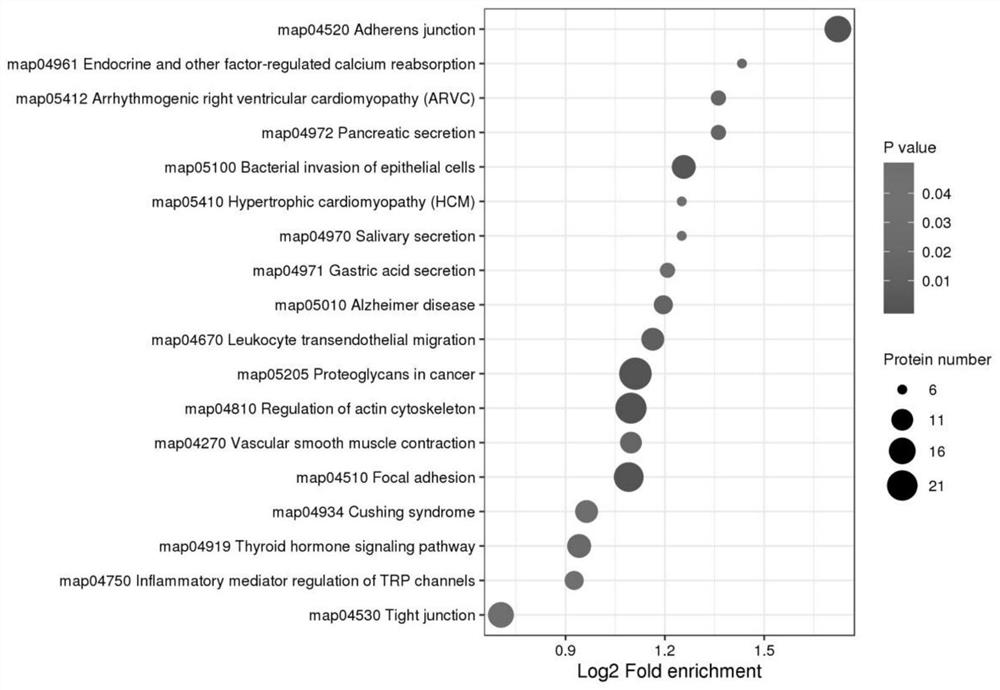
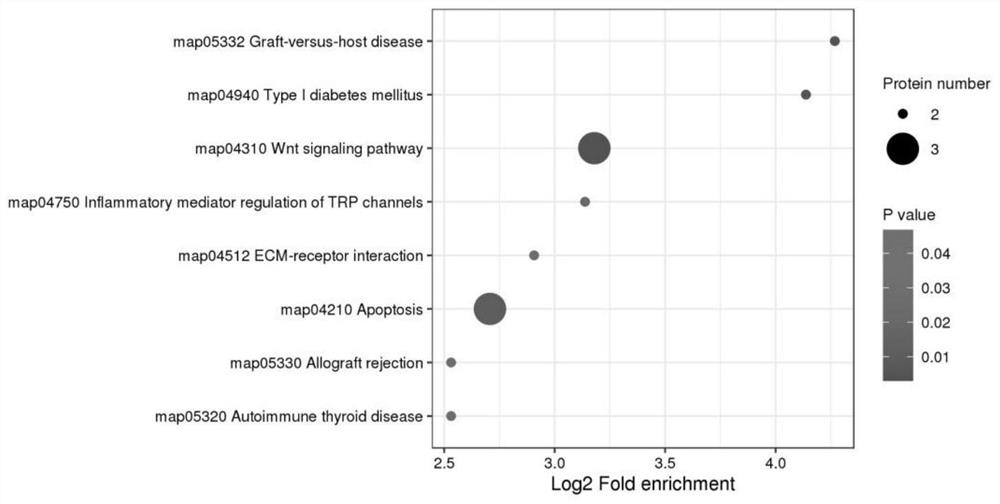
Marker molecules at different time points in endometrial secretion period and screening method thereof
A technology of endometrium and secretory phase, which is applied in the biological field, can solve the problem of not being able to know the molecular changes of the endometrium in the secretory phase, so as to reduce the burden on patients and social pressure, and improve the success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1 protein extraction
[0090] Take the sample out from -80°C, weigh an appropriate amount of tissue sample into a mortar pre-cooled with liquid nitrogen, add liquid nitrogen to fully grind to powder, and add 4 times the volume of sample lysis buffer (1% Triton X-100, 1% protease inhibitor, 1% phosphatase inhibitor) for ultrasonic lysis; 4°C, 12000g centrifugation for 10 min to remove cell debris, transfer the supernatant to a new centrifuge tube, and use the BCA kit for protein concentration determination.
Embodiment 2
[0091] Example 2 trypsin hydrolysis
[0092] Take an equal amount of protein from each sample in Example 1 for enzymatic hydrolysis, adjust the volume to the same volume with the lysate, slowly add 20% TCA at a final concentration, vortex and mix, precipitate at 4°C for 2 hours, centrifuge at 4500g for 5 minutes, and discard the supernatant , wash the precipitate with pre-cooled acetone for 2-3 times, dry the precipitate, add TEAB with a final concentration of 200mM, ultrasonically break up the precipitate, add trypsin at a ratio of 1:50 (protease: protein, m / m), and enzymolyze Overnight, add dithiothreitol (DTT) to make the final concentration 5mM, reduce at 56°C for 30min, then add iodoacetamide (IAA) to make the final concentration 11mM, and incubate at room temperature for 15min in the dark.
Embodiment 3
[0093] Example 3 For phosphorylation modification proteomics, modification enrichment
[0094] Dissolve the peptides obtained in step (2) in the enrichment buffer solution (50% acetonitrile / 0.5% acetic acid), transfer the supernatant to the pre-washed IMAC material, place it on a rotary shaker and incubate with gentle shaking, After the incubation, wash the material three times with buffer solution 50% acetonitrile / 0.5% acetic acid and 30% acetonitrile / 0.1% trifluoroacetic acid in sequence, and finally use 10% ammonia water to elute the phosphopeptide, collect the eluate and dry it by vacuum freeze, After drying, desalt according to the instructions of C18ZipTips, vacuum freeze and dry for liquid mass spectrometry analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com