Novel flavone hydroxylase, microorganism for synthesizing flavone C-glycoside compound and application of novel flavone hydroxylase
A technology of flavone hydroxylase and hydroxyflavanone, applied in the field of synthetic biology and medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
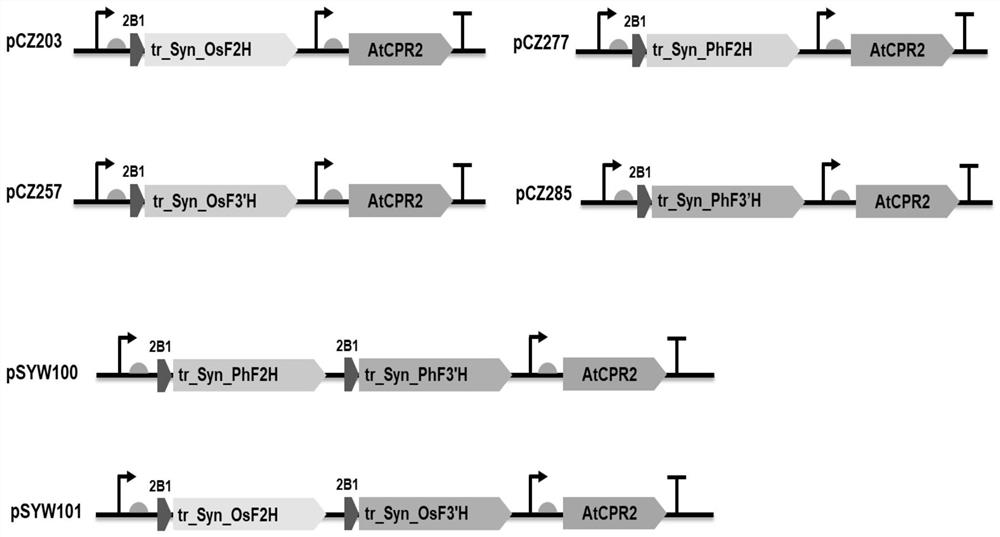
[0112] Embodiment 1, novel cytochrome P450 hydroxylase
[0113] The present invention obtains two novel cytochrome P450 hydroxylases by mining the genome information of Phyllostachys pubescens, the amino acid sequences of which are as follows:
[0114] >PhF2H (SEQ ID NO:1; the underline is the N-terminal transmembrane region)
[0115] m AMELVALVLAAMLLLTVIMRRYG GSAGSSKHGRLPPSPMALPFIGHLHLIRPPPHRAFDRIIARHGPLIYLRLGPSTHCVVVGSADVARDFLRFEASIPERPLTAVTRQLAYGSAGFAFAPYGPHWRFMKRLCMSELLGPRTVEQLRPVRDAELAGVLRAVRAAAERGESVDMSRELIRMSNNAIMRMVASALPGDMAEAARDCAKQVAELVGAFNVEDYVAMCRGWDLQGIGRRTREVHARFDALLETMIRAKEEARRDPLPRDKSRTTKDLLDILMDVAEDEAAEVRLTRENIKAFVLDIFTAGSDTTATSVEWMLAELINHPAYLEKLRAEVDAVVGGSRLVGEPDVAQMPYLQAVLKETLRLRPPAVFAQRETIEAVHVCGYTIPPKTSVFFNIFSIGRDPACWEDPLEFRPERFMPGGASAGVDPKGQHQQLMPFGSGRRACPGMGLAMQAVPAFLAALVQCFDWAVPLPQGTPLDMEEAEGLVSARKQPLVLVPTQRLRPLPAGAAAALA*
[0116] >PhF3'H (SEQ ID NO:2; the underline is the N-terminal transmembrane region)
[0117] m GDVPLPLLLGSLAVSALVWYVLF RRGGGEGA...
Embodiment 2
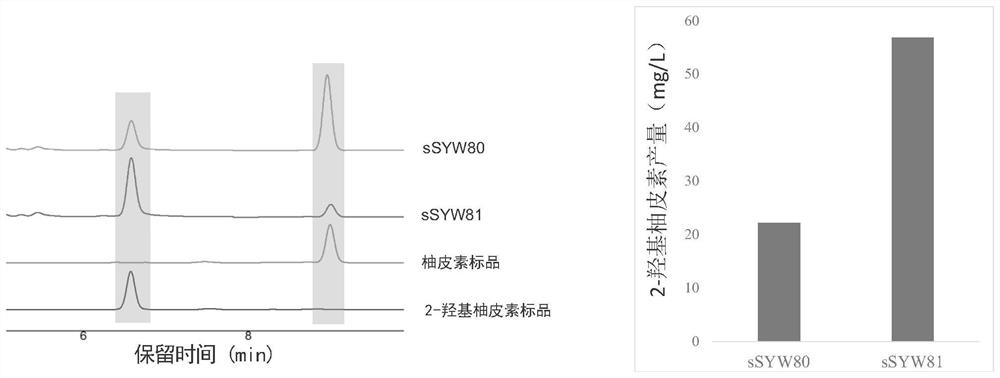
[0119] Embodiment 2, using cytochrome P450 hydroxylase PhF2H to produce 2-hydroxynaringenin
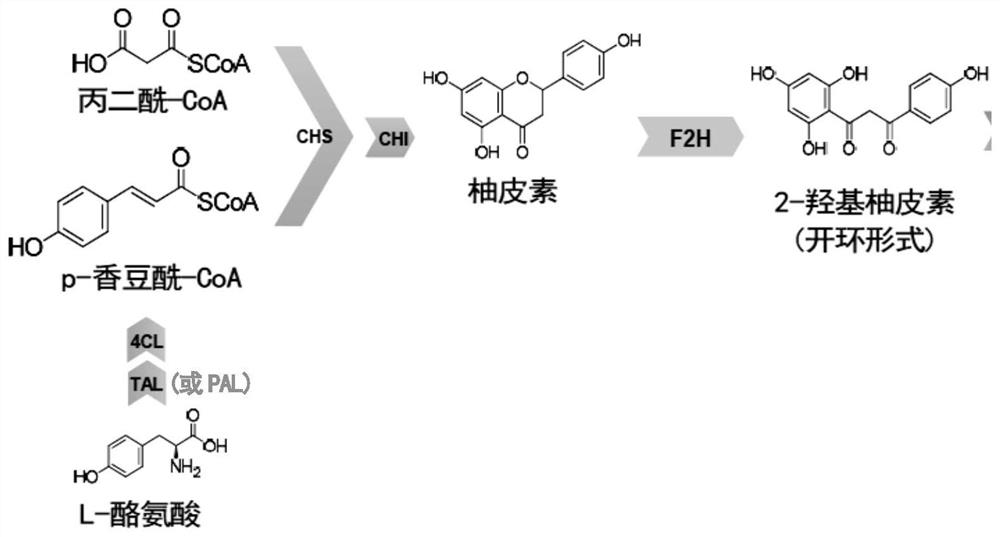
[0120] In this embodiment, the predicted reaction pathway of biosynthetic 2-hydroxynaringenin is as follows figure 1 . It mainly includes: L-tyrosine is catalyzed by aromatic amino acid through tyrosine ammonia lyase (TAL) or phenylalanine ammonia lyase (PAL) and 4-coumaroyl-CoA ligase (4CL) to obtain p - Coumaroyl-CoA, which is catalyzed by chalcone synthase (CHS) and chalcone isomerase (CHI) with malonyl-CoA to obtain naringenin, which is further flavanone-2-hydroxylated Catalyzed by enzyme (F2H), 2-hydroxynaringenin (ring-opened form) is obtained.
[0121] 1. Construction of de novo synthetic naringin plasmid
[0122] The artificially synthesized and codon-optimized (Escherichia coli preference) precursor synthetic gene sequence was constructed on the pET28a vector to obtain pET28-RtPAL, pET28-Pc4CL, pET28-PxhCHS and pET28a-MsCHI, respectively. Among them, PxhCHS: GenBank acces...
Embodiment 3
[0139] Example 3, Cytochrome P450 Hydroxylase (F2H) Combining Carboside Glycosyltransferase to Produce Vitexin, Isovitexin and Their Intermediates
[0140] In this example, vitexin, isovitexin, and dihydroxynaringenin glycosylated products are biosynthesized, and the reaction pathway is as follows Figure 4 . It mainly includes: L-tyrosine is catalyzed by aromatic amino acid through tyrosine ammonia lyase (TAL) or phenylalanine ammonia lyase (PAL) and 4-coumaroyl-CoA ligase (4CL) to obtain p - Coumaroyl-CoA, which is catalyzed by chalcone synthase (CHS) and chalcone isomerase (CHI) with malonyl-CoA to obtain naringenin, which is further flavanone-2-hydroxylated Enzyme (F2H) catalyzes to obtain 2-hydroxynaringenin (ring-opened form); 2-hydroxynaringenin obtains 2-hydroxynaringenin-C-glucoside through glycosyltransferase (CGT), and then dehydrates to form oyster Vitexin or isovitexin.
[0141] 1. Construction of de novo synthetic naringin plasmid
[0142] With embodiment 2. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com