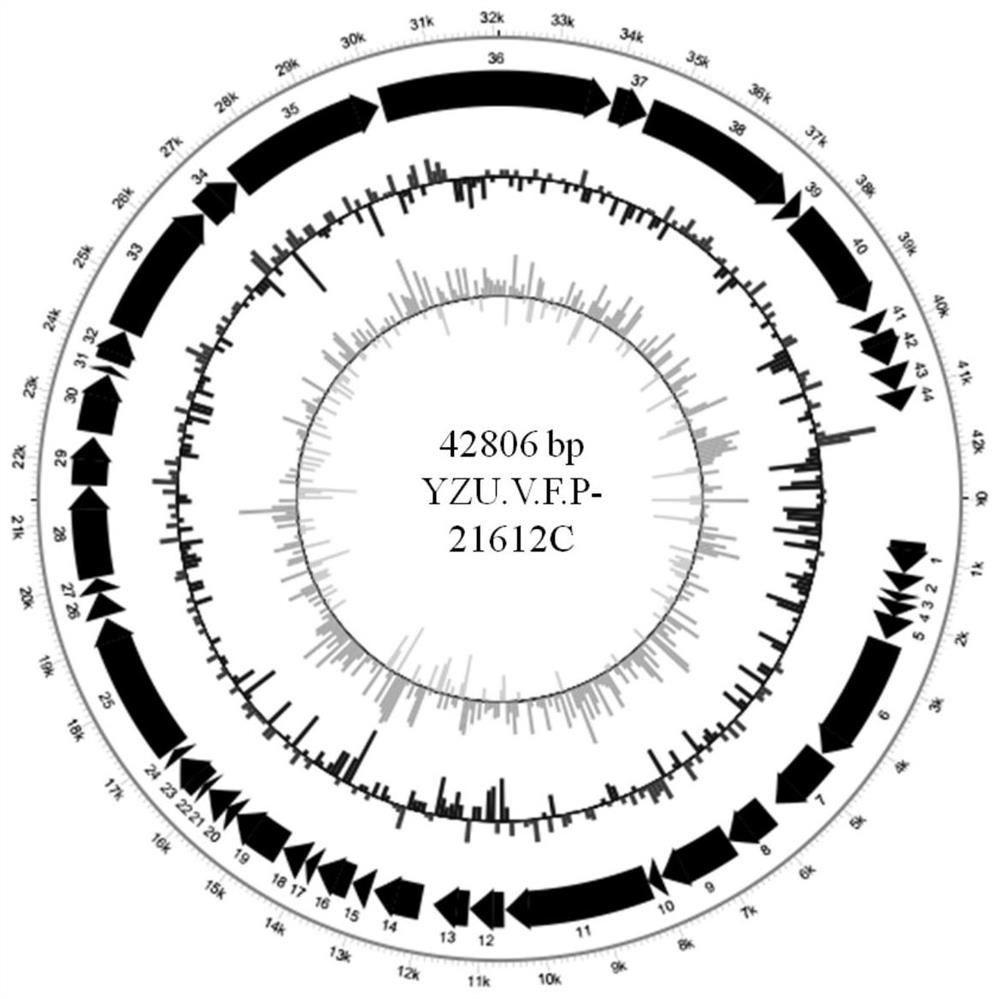
Vibrio fluvialis bacteriophage YZU.V.F.P-21612C and application thereof
A technology of YZU.V.F.P-21612C and Vibrio riverina, which is applied in the field of bacteriostasis and enterobacter phage, can solve the problems of insufficient research and insufficient research on bacteriostatic agents, so as to reduce pollution, reduce transmission and cause Effect of Foodborne Illness Risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
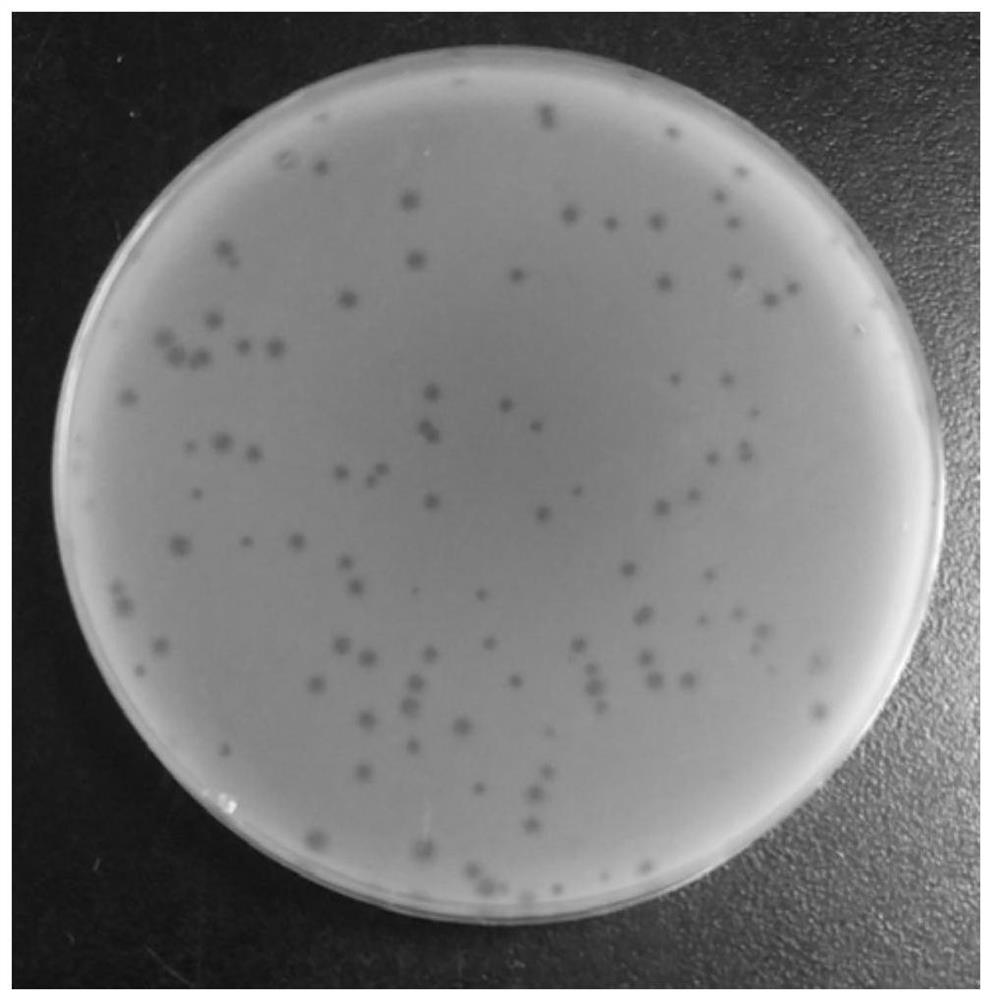
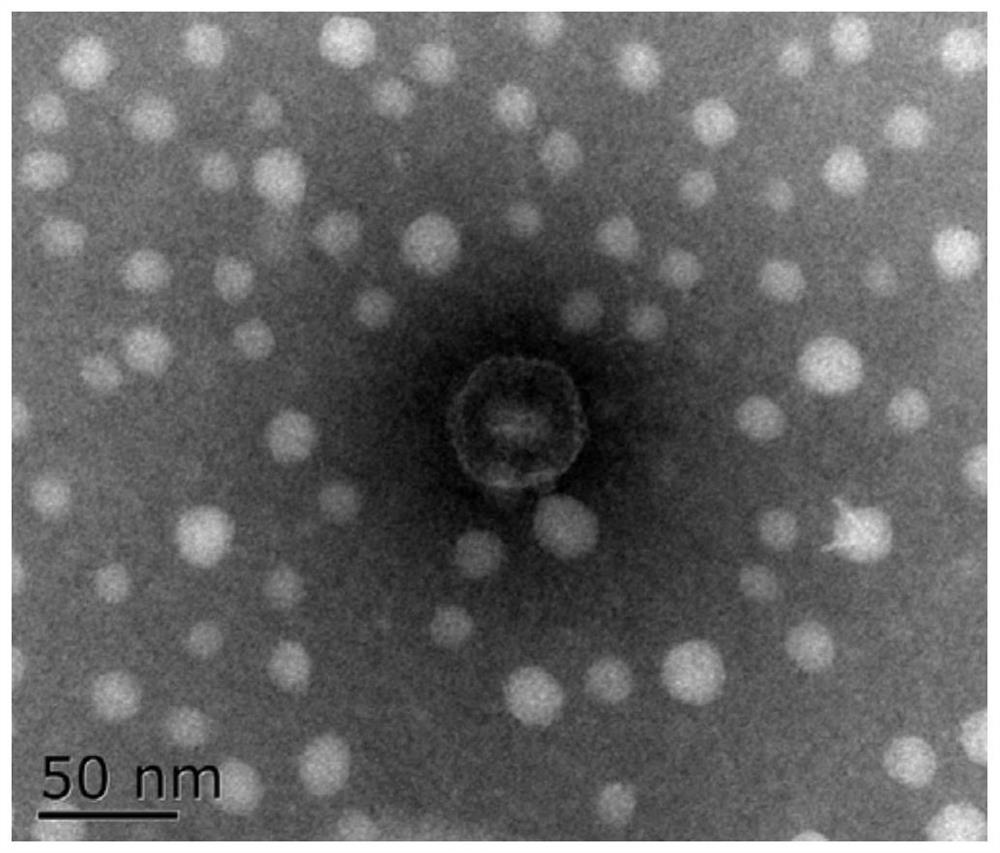
[0036] Phage isolation and purification preparation
[0037] Phage isolation
[0038] Sewage samples were collected from the farmer's market in Yangzhou City, Jiangsu Province. Take 30mL sewage sample into a 50mL centrifuge tube, centrifuge at 5000×g for 10min, take 5mL supernatant and add it to 5mL LBS liquid medium, and add 100μL of Vibrio fluviobacter strain CICC21612 in the logarithmic growth phase at the same time, 37°C, 100rpm shaker Cultivate overnight; transfer the culture in the test tube to a sterile centrifuge tube, centrifuge at 5000×g for 10 min at 4°C, and pass the supernatant through a 0.22 μm filter membrane to obtain the phage stock solution.
[0039] Use SM buffer (1L: NaCl 5.8g, MgSO4.7H2O 2.0g, 1M Tris-HCl (pH7.4) 50mL) to carry out 10 gradient dilutions of the phage stock solution according to the volume ratio, and take 100 μL of the diluted phage suspension and 100 μL logarithmic Mix the vibrio in the growth phase for 10 minutes at room temperature, the...
Embodiment 2
[0049] Bacteriostatic effect of phage YZU.V.F.P-21612C in culture medium
[0050] Vibrio riverine (strain CICC21612) was pressed by 10 5 CFU / mL is inoculated into 30mL LBS liquid culture medium, adds phage YZU.V.F.P-21612C and VmYZU10474 (pathogenic Vibrio phage VmYZU10474 in the patent CN 112063593A) suspension to 10 in the phage treatment group respectively. 9 PFU / mL, an equal volume of SM buffer (0PFU / mL) was added to the negative control group, and the treated inoculum was placed in a 37°C incubator for constant temperature cultivation, and 1 mL was sampled every 2 hours, and the total number of colonies was measured by the coating method , 3 parallels were set for each group, and the average value was used for analysis.
[0051] The implementation results are as follows Figure 4 As shown, as the culture time prolongs, the total number of bacterial colonies in the negative control group increases rapidly, reaching 10 in 12 hours. 8 CFU / mL, while the total number of col...
Embodiment 3
[0053] Inhibitory Effect of Bacteriophage YZU.V.F.P-21612C on Vibrio riverine Biofilm
[0054] Take the Vibrio riverina culture (Vibrio riverina CICC21612 bacterium liquid) in the logarithmic growth phase, dilute it with LBS medium, and adjust the final concentration to be 1×10 7 CFU / mL. The test was divided into YZU.V.F.P-21612C treatment group, negative control group and blank group. YZU.V.F.P-21612C treatment group: Add 150 μL to each well of a 96-well plate with a concentration of 10 7 CFU / mL Vibrio riverina (CICC21612) bacterial liquid and 150 μL phage YZU.V.F.P-21612C suspension (A treatment was phage 105 PFU / mL, B treatment is 10 6 PFU / mL, C treatment is 10 7 PFU / mL, D treatment is 10 8 PFU / mL, E treatment is 10 9 PFU / mL); Negative control group: Add bacterial solution (10 7 CFU / mL) and 150 μL each of SM buffer; blank group: add 300 μL LBS medium to each well; incubate at 37°C for 24 hours, take out the 96-well plate, suck out the suspended bacteria, wash 3 times ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com