Anti-tumor engineering exosome, preparation method and application
An exosome, anti-tumor technology, used in biochemical equipment and methods, anti-tumor drugs, genetic engineering, etc., can solve the problems of limiting the application and transformation of siRNA, and low gene silencing efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
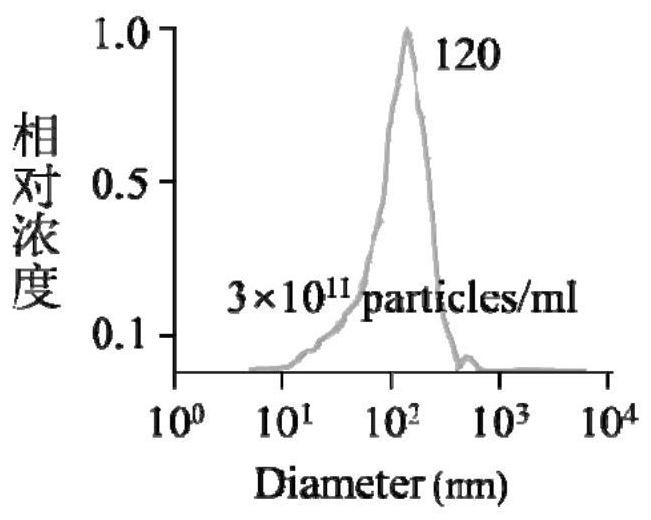
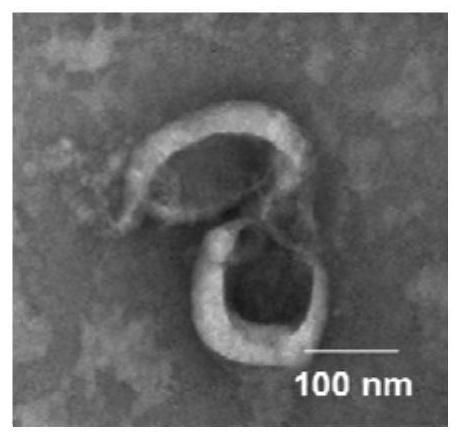
[0033] The extraction and identification of embodiment 1 Nex
[0034] Culture NK92MI cells (purchased from the Cell Bank of the Chinese Academy of Sciences) with exosome-free medium or serum-free medium. When the cells are in the logarithmic growth phase, centrifuge at 300g for 10 minutes to collect the supernatant (the supernatant can be stored at -80°C for one week) ).
[0035]The above supernatant (thawed in cold water if taken out from -80°C) was performed as follows: centrifuge at 2000g for 15 minutes to remove dead cells, collect the supernatant and discard the precipitate; centrifuge at 10000g for 30 minutes to remove cell debris, collect the supernatant and discard Precipitation; filter the supernatant with a 0.22 μm filter membrane to remove microvesicles with larger particle sizes. The obtained filtrate was transferred to a 100kD ultrafiltration tube, centrifuged at 3000g for 20 minutes, and the lower layer filtrate was discarded to obtain a culture supernatant conc...
Embodiment 2
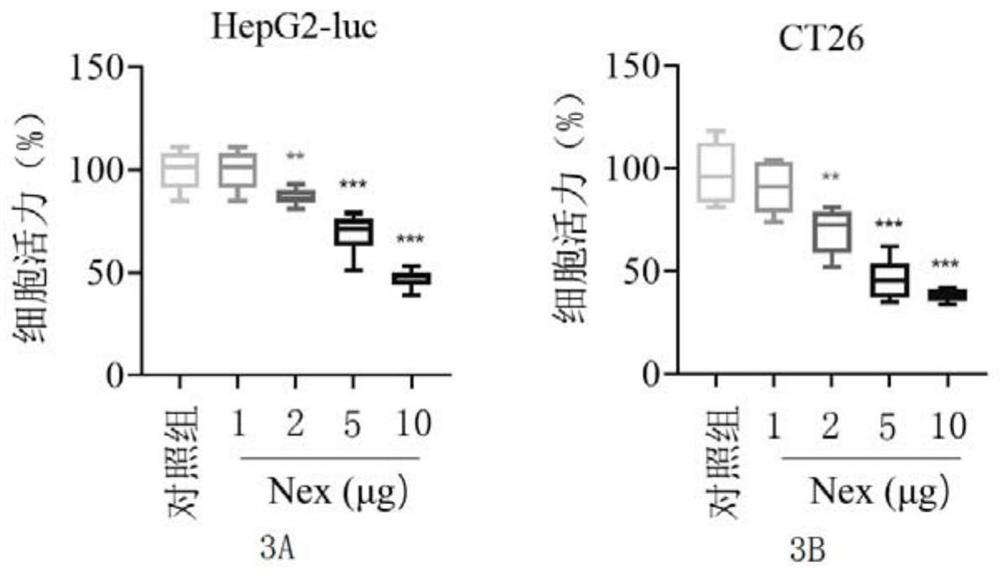
[0037] Example 2 Anti-tumor activity of Nex at the cellular level
[0038] HepG2-luc and CT26 cells (purchased from the Cell Bank of the Chinese Academy of Sciences) were trypsinized and resuspended in DMEM medium and RPMI-1640 medium (Gibco) respectively, so that the cell density was about 1×10 5 individual / mL. Add 100 μL of cell suspension to each well of the 96-well plate, and continue to store at 37°C, 5% CO 2 cultured in a cell culture incubator for 24 hours. Add different doses of Nex to the cells, and after 48 hours of treatment, add an equal amount of MTT solution (Sigma, also known as tetrazolium salt) to the 96-well plate, incubate at 37°C for 4 hours, remove the medium containing MTT, and wash with PBS 3 times. Add 100 μL DMSO solution (dimethyl sulfoxide solution) to each well and let stand at room temperature for 5-10 minutes. The absorbance values at 540nm and 650nm were recorded respectively with a microplate detector, and the cell survival rate was calcul...
Embodiment 3
[0044] Example 3 Preparation of Nex@siRNA / Ce6 and analysis of cell entry effect
[0045] The specific steps are:
[0046] (1) Take 100 μL of the purified Nex suspension (1 mg / mL) obtained in Example 1 into a 1.5 mL EP tube and place it on ice.
[0047] (2) PLK1 (Polo-like Kinase 1, Polo-like Kinase 1) siRNA dry powder (siPLK1, Suzhou Ruibo Biotechnology Co., Ltd.) was dissolved in DEPC (diethylpyrocarbonate) water to make the concentration 1 mg / mL.
[0048] (3) Take 100 μL of siPLK1 solution and add it to Nex, pipette gently to evenly transfer 200 μL of the mixture to a pre-cooled 0.4 cm electroporation cup.
[0049] (4) Add 200 μL of PBS to the electroporation cup, and gently pipette evenly to make the total volume of the system 400 μL.
[0050] (5) Use the Bio-RAD electroporator for electroporation, and set the electroporation parameters as: 200V, 125μF, 200Ω.
[0051] (6) Aspirate the mixed solution from the electroporation cup and transfer it to a new EP tube, and incub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com