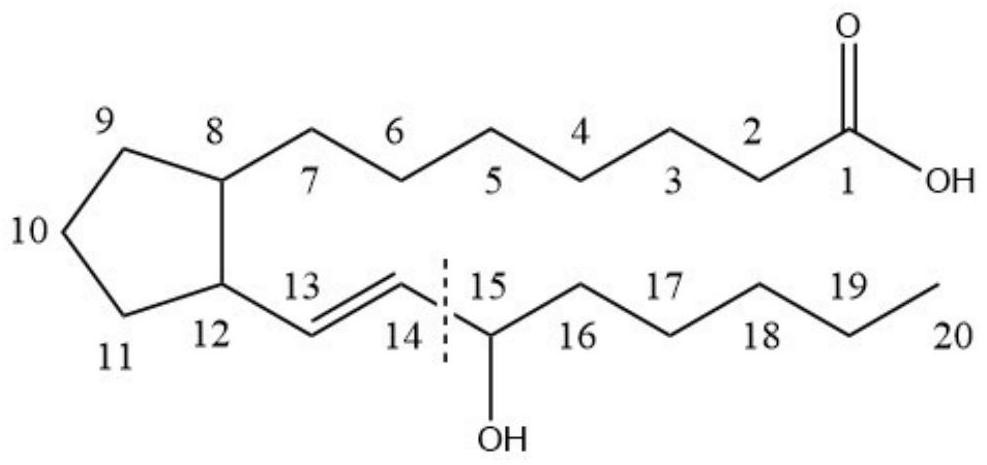
Method for detecting prostaglandin substances in biological sample
A technology for prostaglandins and biological samples, applied in the field of quantitative analysis of a series of prostaglandins in biological samples, can solve problems such as difficulty in carrying out research work, and achieve the effects of reducing the amount of biological samples, simplifying sample processing steps, and shortening retention time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
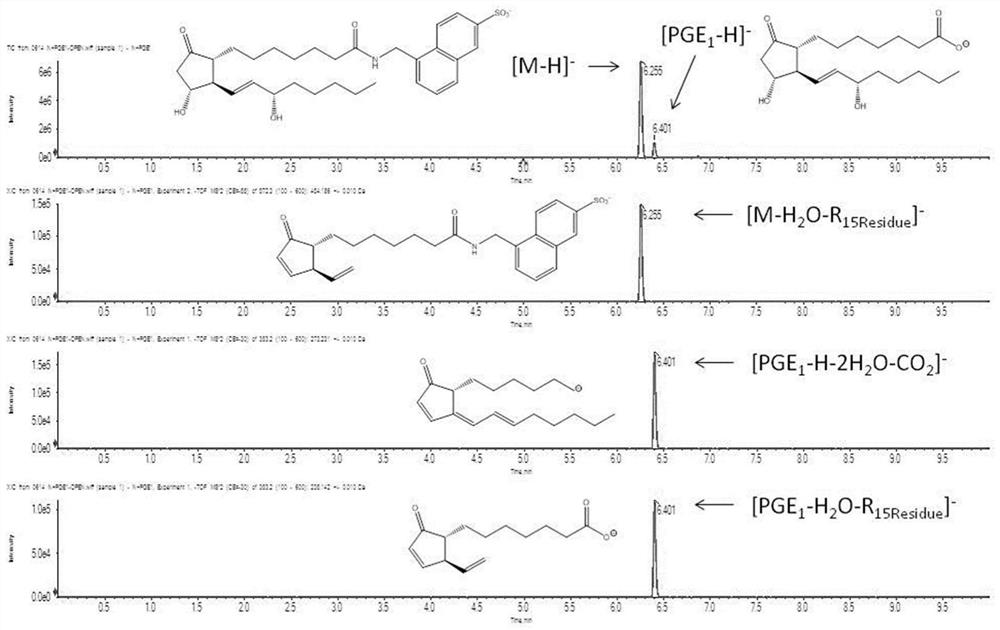
[0034] Add 1mL of acetonitrile / water mixture into the anti-adsorption EP tube, and add 1mg / mL PGE in sequence 1 Acetonitrile solution 20 μL, 1 μL / mL DIPEA acetonitrile solution 50 μL, 1 mg / mL HATU acetonitrile solution 50 μL, shake at room temperature for 10 minutes; add 1 mg / mL 5-(aminomethyl)naphthalene-2-sulfonic acid aqueous solution 50 μL, shake at room temperature Shake for 20 minutes.
[0035] After the reaction was completed, 50 μL of the reaction mixture was taken out from the reaction solution and added to 4950 μL of acetonitrile water 1 / 1 (v / v) to dilute into the test solution. Mass spectrometry not found [PGE 1 -H] - signal (m / z 353.23Da), and the corresponding PGE 1 The signal of benzenesulfonic acid derivative is obvious (m / z 572.27Da), indicating that PGE 1 Fully derivatized.
[0036] Triple TOF TM 6600 + Type tandem mass spectrometer, equipped with ESI ionization source; negative ion detection; ion injection voltage -5000V; temperature 500°C; source gas...
Embodiment 2
[0042] According to the derivatization scheme described in Example 1, the equivalent amount of limatoprost was converted into 4-(aminomethyl)benzenesulfonic acid derivatives; and the mass spectrometry conditions were optimized.
[0043] Column: ACQUITY TM BEH C 18 Column, 100mm×2.1mm I.D., 1.7μm particle size; mobile phase: aqueous phase: 5% acetonitrile + 95% water (containing 1mMol ammonium acetate + 0.02% acetic acid) pH 5, organic phase: acetonitrile; gradient elution; column The temperature is 40°C; the flow rate is 0.3mL / min; the injection volume is 20μL. The gradient elution in the chromatographic conditions, the program is shown in Table 2, wherein A is 5% acetonitrile + 95% water (containing 1mMol ammonium acetate + 0.02% acetic acid), and B is acetonitrile.
[0044] Table 2 Gradient elution program
[0045]
[0046] The mass spectrometry conditions are: Triple Quad TM Model 6500 tandem mass spectrometer, equipped with ESI ionization source, SelexION differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com