Separation and purification method of 2-(4-fluorophenyl)thiophene
A purification method, fluorophenyl technology, applied in the field of separation and purification of 2-thiophene, can solve the problems of long time consumption, low melting point, high energy consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Synthesis of 2-(4-fluorophenyl)thiophene:
[0024] in N 2 Under protection, add 1.63g of 2-bromothiophene, 25.9mg of 1,2-bis(diphenylphosphine)ethane nickel chloride, and 2.1mL of anhydrous tetrahydrofuran into the round bottom flask, cool down to t=2°C, stir, Add 10mL of 1M / L 4-fluorophenylmagnesium bromide (THF solution) dropwise, and control the temperature T≤30°C; then stir at 22°C for 1.5h, and quench the reaction solution with dilute hydrochloric acid.
[0025] (2) Separation and purification of 2-(4-fluorophenyl)thiophene:
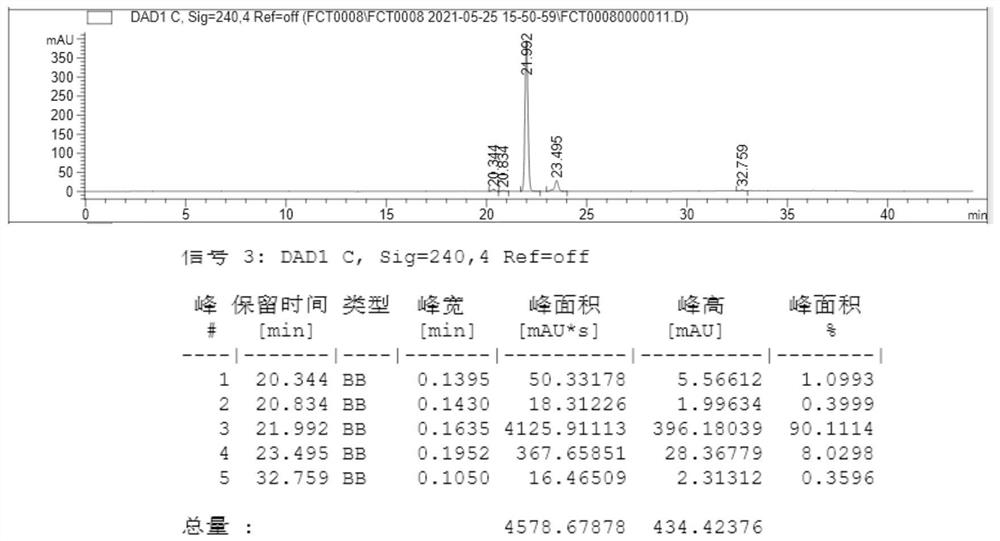
[0026] The mixture obtained in the above reaction was extracted with n-heptane to extract the organic phase, dried over anhydrous sodium sulfate, filtered with diatomaceous earth, and the filtrate was collected. The HPLC spectrum of the filtrate is as follows: figure 1 Shown; Add organic solvent acetonitrile, stir at room temperature for 4h, leave to stand for 2h and then separate layers, the upper layer is a n-heptane phase, and its HP...
Embodiment 2
[0028] (1) Synthesis of 2-(4-fluorophenyl)thiophene:
[0029] in N 2 Under protection, add 1.63g of 2-bromothiophene, 25.9mg of 1,2-bis(diphenylphosphine)ethane nickel chloride, and 2.1mL of anhydrous tetrahydrofuran into the round bottom flask, cool down to t=2°C, stir, Add 10mL of 1M / L 4-fluorophenylmagnesium bromide (THF solution) dropwise, and control the temperature T≤30°C; then stir at 22°C for 1.5h, and quench the reaction solution with dilute hydrochloric acid.
[0030] (2) Separation and purification of 2-(4-fluorophenyl)thiophene:
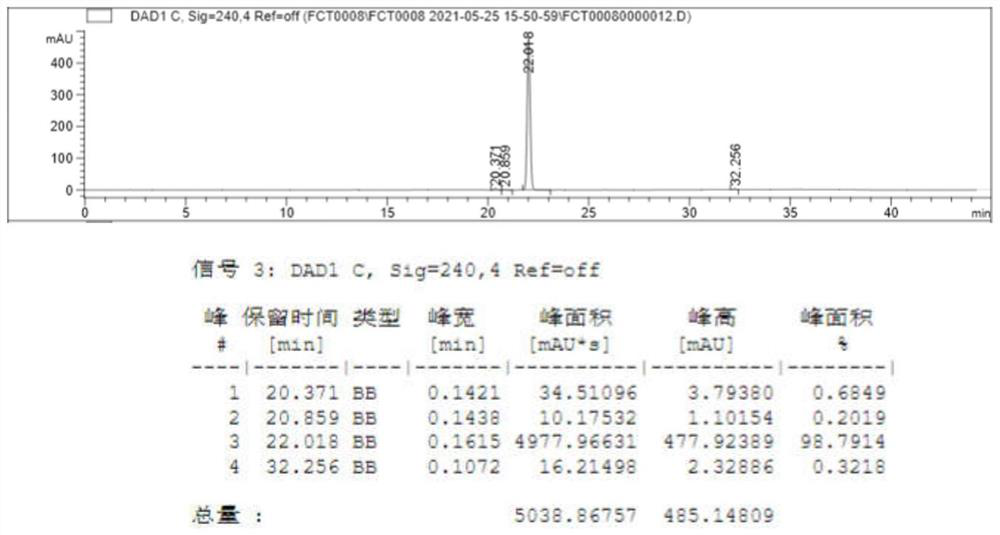
[0031] Extract the organic phase of the above reaction mixture with cyclohexane, dry over anhydrous sodium sulfate, filter with diatomaceous earth, collect the filtrate, add the organic solvent acetonitrile, stir at room temperature for 3 hours, and separate layers after standing for 4 hours. The upper layer is the n-heptane phase, and the lower layer It is the acetonitrile phase, and the upper layer is taken by liquid separation, and t...
Embodiment 3
[0033] (1) Synthesis of 2-(4-fluorophenyl)thiophene:
[0034] in N 2 Under protection, add 1.63g of 2-bromothiophene, 25.9mg of 1,2-bis(diphenylphosphine)ethane nickel chloride, and 2.1mL of anhydrous tetrahydrofuran into the round bottom flask, cool down to t=2°C, stir, Add 10mL of 1M / L 4-fluorophenylmagnesium bromide (THF solution) dropwise, and control the temperature T≤30°C; then stir at 22°C for 1.5h, and quench the reaction solution with dilute hydrochloric acid.
[0035] (2) Separation and purification of 2-(4-fluorophenyl)thiophene:
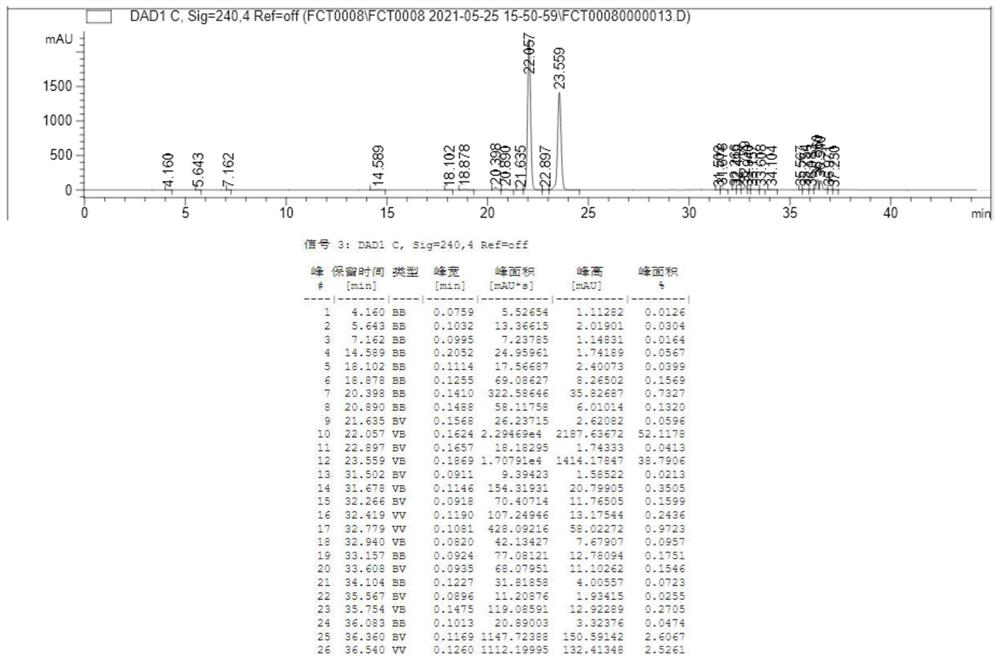
[0036] Extract the organic phase of the above reaction mixture with cyclopentane, dry over anhydrous sodium sulfate, filter with diatomaceous earth, collect the filtrate, add organic solvent acetonitrile, stir at room temperature for 5 hours, and separate layers after standing for 3 hours. The upper layer is the n-heptane phase, and the lower layer It is the acetonitrile phase, and the upper layer is taken by liquid separation, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com