Synthesis method of 9,10-anthracenedicarboxylic acid
A technology for the synthesis of anthracene dicarboxylic acid, applied in chemical instruments and methods, preparation of carboxylate salts, preparation of organic compounds, etc., capable of solving the problems of cumbersome synthesis routes and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Add 1.00 g, 0.6 mmol of anthracene in a 100 mL round bottom flask, then add 20 mL of 48% hydrogen bromide in aqueous solution, then add 5 mL of acetic acid solution, slowly add 1.0 g, 12.8 mmol of paraformaldehyde and 0.04g, 0.128mmol N,N,N-trimethyl-1-tetradecylammonium bromide, heated to 45~55℃ and heated under N 2 React under protection for 24 to 48 hours. After the reaction is completed, cool to room temperature to produce a large amount of solids, filter and wash the filter residue with water and ethanol, and dry to obtain the first intermediate;
[0026] (2) Add 1.99g, 5.5mmol of the first intermediate to a 100mL round bottom flask, then add 20mL of dry dimethyl sulfoxide (DMSO) and stir to dissolve, accurately measure 20mL of sodium ethoxide solution and 2mL of 2-nitropropane The mixed solution was slowly added dropwise into a round-bottomed flask in a constant pressure dropping funnel, and reacted at room temperature for 24 to 36 hours. After the reaction, t...
Embodiment 2
[0034] The difference between the present embodiment and Example 1 is: in step (1), the mol ratio of paraformaldehyde and N,N,N-trimethyl-1-tetradecyl ammonium bromide is 95:1; step ( In 2), the eluent in the chromatography column is PE:EA=95:1; in step (3), the molar ratio of sulfamic acid and sodium chlorite is 1:1.2.
Embodiment 3
[0036] The difference between the present embodiment and embodiment 1 is: in step (1), the mol ratio of paraformaldehyde and N,N,N-trimethyl-1-tetradecyl ammonium bromide is 105:1; In step (2), the eluent in the chromatography column is PE:EA=105:1.
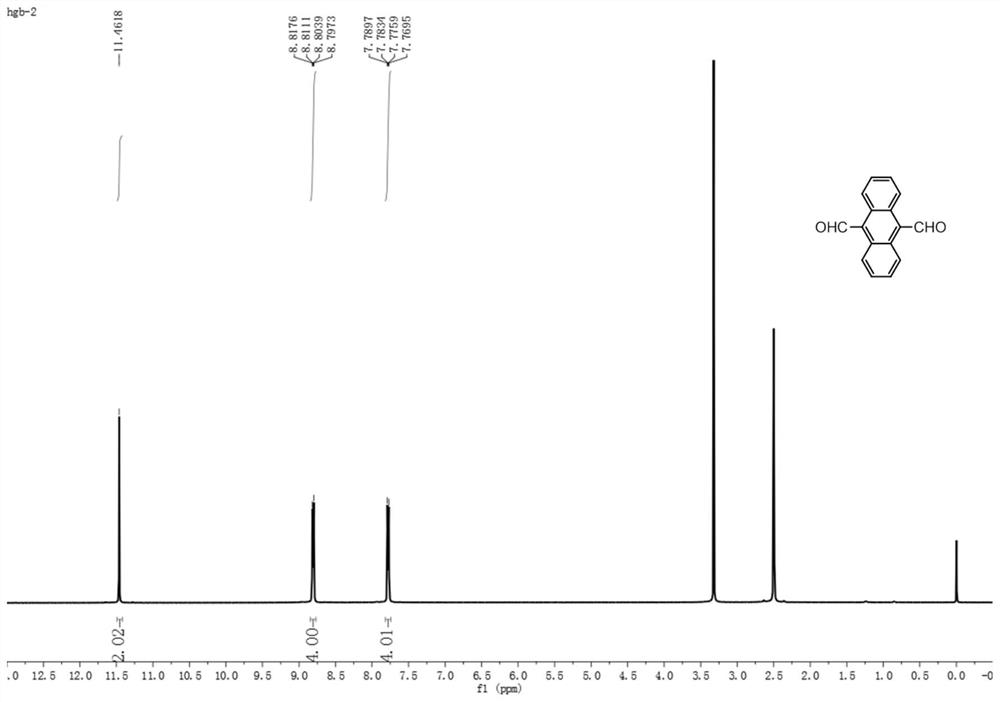
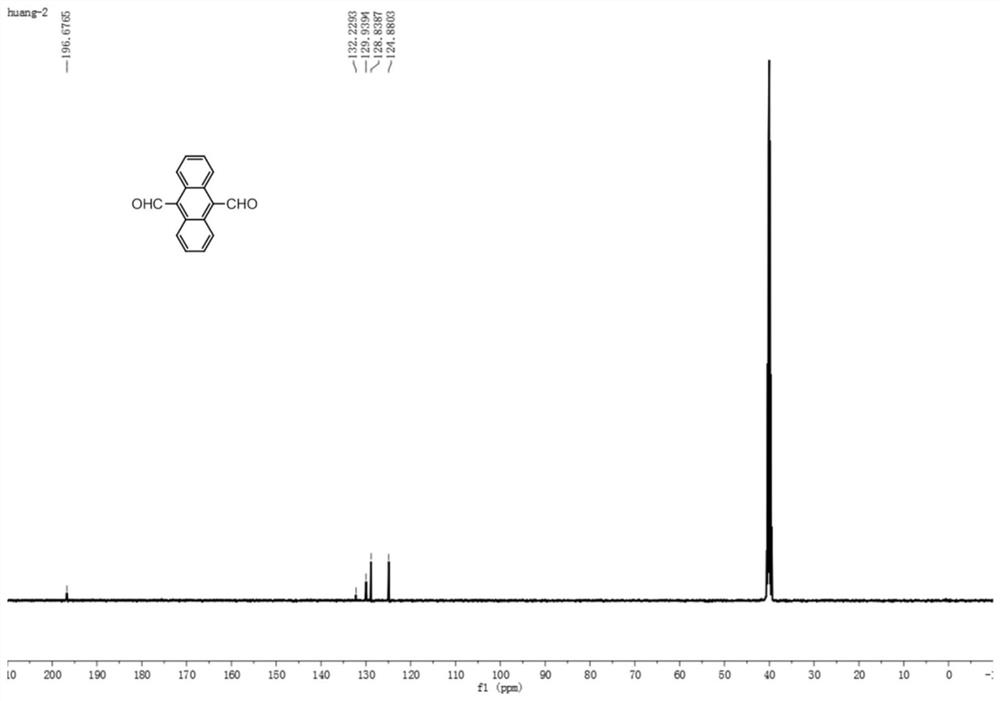
[0037] The second intermediate 9,10-dialdehyde group anthracene prepared in Example 1 is an orange solid with a yield of 60%, such as figure 1 , figure 2 as shown, 1 H NMR nuclear magnetic resonance analysis results are 1 H NMR (500MHz, DMSO) δ11.46(s, 2H), 8.81(dd, J=6.9, 3.3Hz, 4H), 7.78(dd, J=6.9, 3.2Hz, 4H). 13 C NMR nuclear magnetic resonance analysis results are 13 C NMR (126 MHz, DMSO) δ 196.7, 132.2, 129.9, 128.8, 124.9.
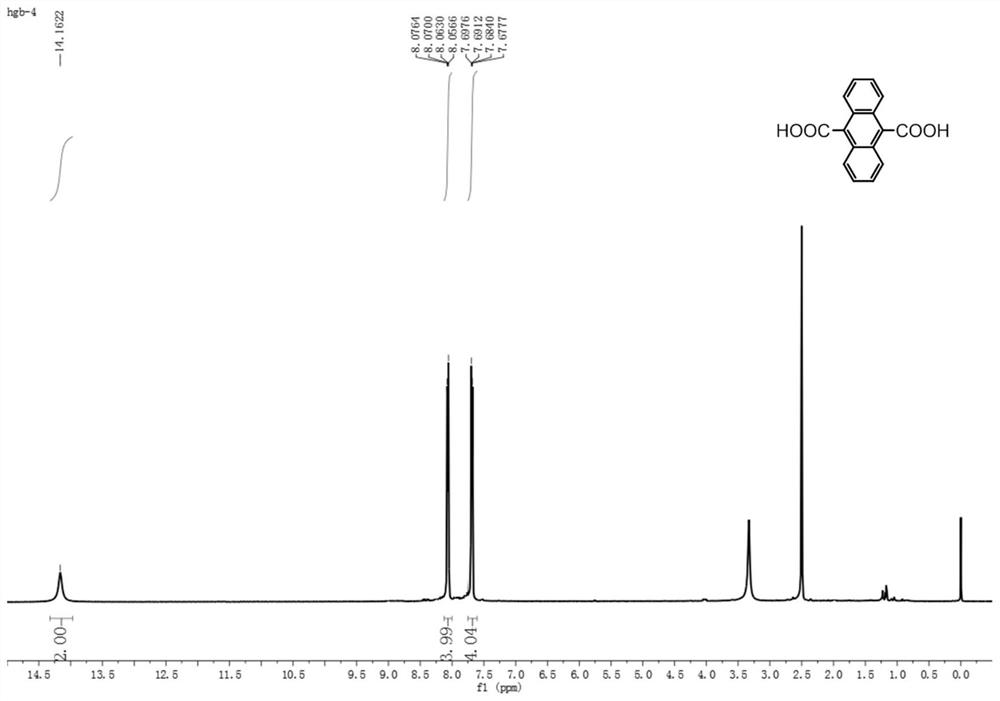
[0038] The 9,10-anthracene dicarboxylic acid prepared in Example 1 is an orange solid with a yield of 60%, as shown in image 3 , Figure 4 , Figure 5 as shown, 1 H NMR nuclear magnetic resonance analysis results are 1 H NMR (500MHz, DMSO) δ 14.16 (s, 2H), 8.07 (dd, J=6.7, 3.2Hz, 4H), 7.69 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com