Preparation method of sodium picosulfate
A technology of sodium picosulfate and acidic conditions, which is applied in the field of preparation of sodium picosulfate, and can solve problems such as difficulty in purification, slow reaction speed, and reduced oxidation of concentrated sulfuric acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
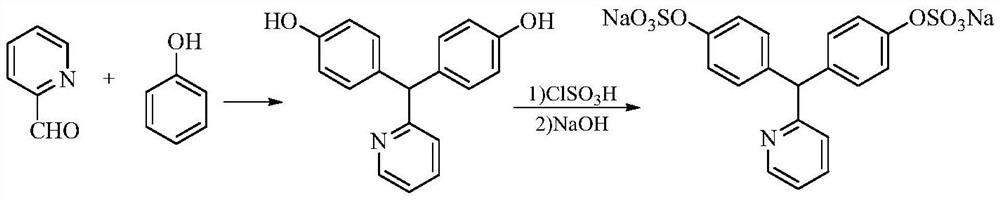
[0041] 1. Synthesis of 4,4'-(2-pyridinemethylene)-bisphenol
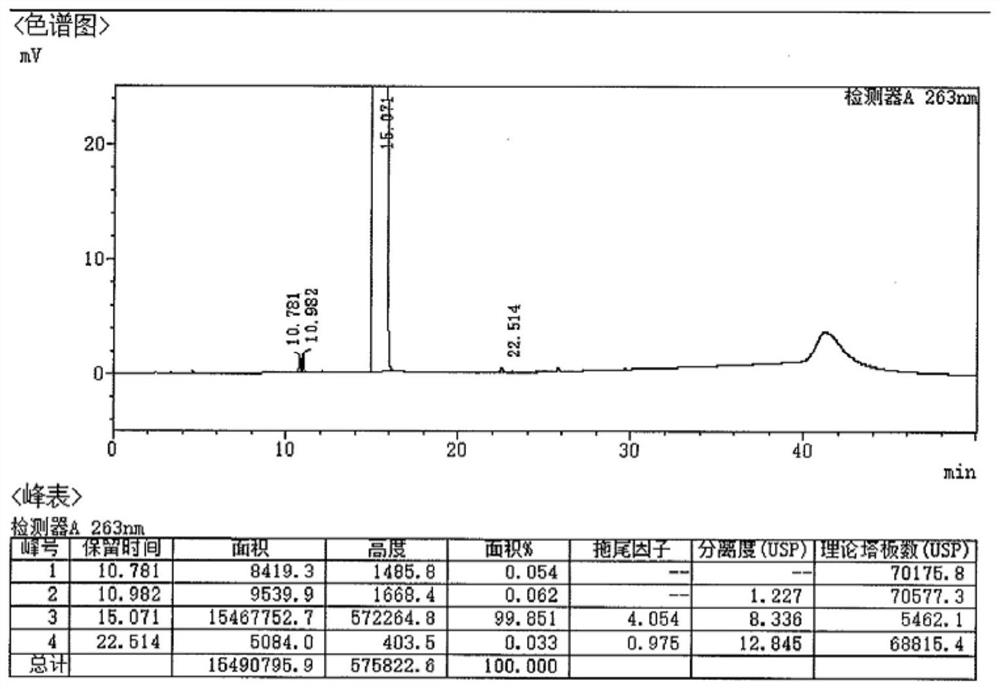
[0042] Phenol (79.1g, 0.84mol), pyridine-2-carbaldehyde (42.9g, 0.4mol), 79ml of hydrochloric acid, and 100ml of glacial acetic acid were successively added to a 1L three-necked flask, heated to 40-50°C and stirred for 10 hours, then concentrated under reduced pressure to Dry, cool down to 0-10°C, slowly add 30% sodium hydroxide solution dropwise, adjust pH = 7-7.5, add 500ml absolute ethanol dropwise, a large amount of solid precipitates, filter, wash with purified water, methanol and ethyl acetate Esters (volume ratio: 1:8) were recrystallized in a mixed solvent to obtain 72.4 g of a white solid, yield: 65.3%, isomer impurity 2,4'-(pyridin-2-ylmethylene)-bisphenol 0.21 %.
[0043] 2. Synthesis of sodium picosulfate
[0044]400ml of pyridine, 2.8g of morpholine, 7ml of N-methylpyrrolidone, 4,4'-(2-pyridylmethylene)-bisphenol (69.3g, 0.25mol), sulfamic acid ( 68.9g, 0.71mol), heated to 40-50°C and stirred for 12 ...
Embodiment 2
[0046] 1. Synthesis of 4,4'-(2-pyridinemethylene)-bisphenol
[0047] Phenol (103.5g, 1.1mol), pyridine-2-carboxaldehyde (53.6g, 0.5mol), 100ml hydrochloric acid, and 130ml glacial acetic acid were successively added to a 500ml three-necked flask, heated to 40-50°C and stirred for 8 hours, then concentrated under reduced pressure to Dry, cool down to 0-10°C, slowly add 30% sodium hydroxide solution dropwise, adjust pH=6-7, add 650ml absolute ethanol dropwise, precipitate a large amount of solid, filter, wash, use methanol and ethyl acetate Recrystallize according to the mixed solvent with a volume ratio of 1:8 to obtain 87.6g of white solid, yield: 63.2%, isomer impurity 2,4'-(pyridin-2-ylmethylene)-bisphenol 0.17% .
[0048] 2. Synthesis of sodium picosulfate
[0049] 700ml of pyridine, 5.6g of morpholine, 20ml of N-methylpyrrolidone, 4,4'-(2-pyridylmethylene)-bisphenol (138.7g, 0.5mol), sulfamic acid ( 140.8g, 1.45mol), heated to 40-50°C and stirred for 12 hours. Add 3.5L...
Embodiment 3
[0051] 1. Synthesis of 4,4'-(2-pyridinemethylene)-bisphenol
[0052] Add phenol (210.8g, 2.24mol), pyridine-2-carbaldehyde (107.1g, 1.0mol), 200ml hydrochloric acid, and 250ml glacial acetic acid to a 1000ml three-necked flask in sequence, heat up to 40-50°C and stir for 8 hours, then concentrate under reduced pressure to Dry, cool down to 0-10°C, slowly add 30% sodium hydroxide solution dropwise, adjust pH=6-7, add dropwise 1300ml of absolute ethanol-ethyl acetate mixed solution, precipitate a large amount of solid, filter, wash, Recrystallization was carried out using ethanol and ethyl acetate in a mixed solvent with a volume ratio of 1:8 to obtain 178.8 g of a white solid, yield: 64.5%, isomer impurity 2,4′-(pyridin-2-ylmethylene )-bisphenol 0.19%.
[0053] 2. Synthesis of sodium picosulfate
[0054] 1380ml of pyridine, 10.8g of morpholine, 45ml of N-methylpyrrolidone, 4,4'-(2-pyridylmethylene)-bisphenol (277.3g, 1.0mol), sulfamic acid ( 279.6g, 2.88mol), heated to 40-50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com