A chemical analysis method of copper phase in copper concentrate
A technology of copper concentrate and physical phase, applied in the field of chemical analysis, to avoid inaccurate measurement and ensure the safety of the measurement process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
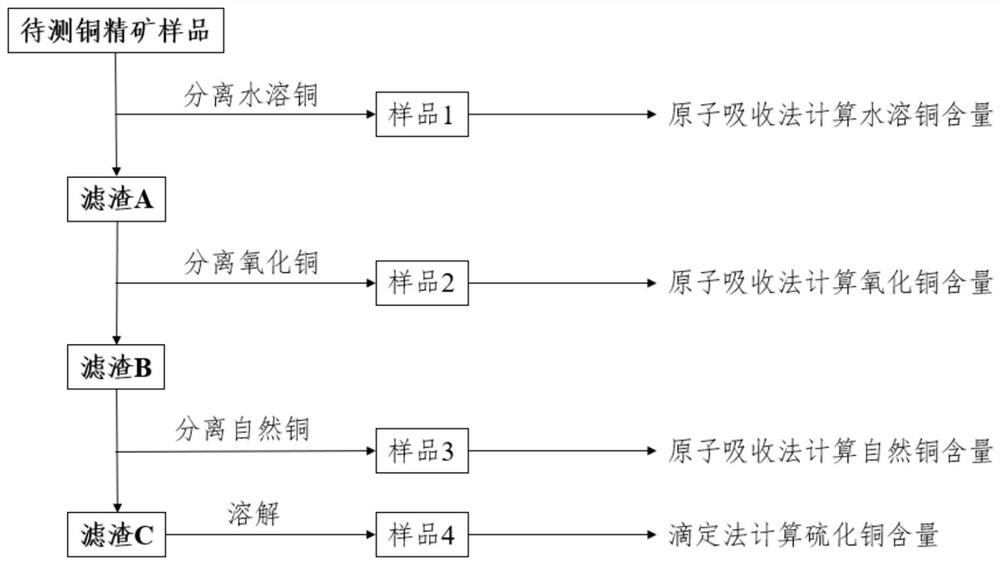
[0046] According to the previous experimental results and the relative significance of various copper substances in smelting and customs supervision, the various copper phases in imported copper concentrates can be divided into four categories, namely water-soluble copper (mainly bile vitriol and water bila vitriol) , copper oxide (mainly copper ore, black copper ore), natural copper, copper sulfide (mainly chalcopyrite, bornite, chalcocite, copper blue). The main experimental steps of the chemical analysis method of copper phase in copper concentrate are as follows:
[0047] 1. After taking a representative sample according to the regulations, after mixing, shrinking, grinding, and passing through a 150-mesh sieve, the sample to be tested is obtained;
[0048] 2. Use X-ray fluorescence spectroscopy (XRF) to qualitatively analyze the elements in the sample to be tested;
[0049] 3. Use X-ray diffraction (XRD) and qualitatively analyze the copper phase in the sample to be test...
Embodiment 2
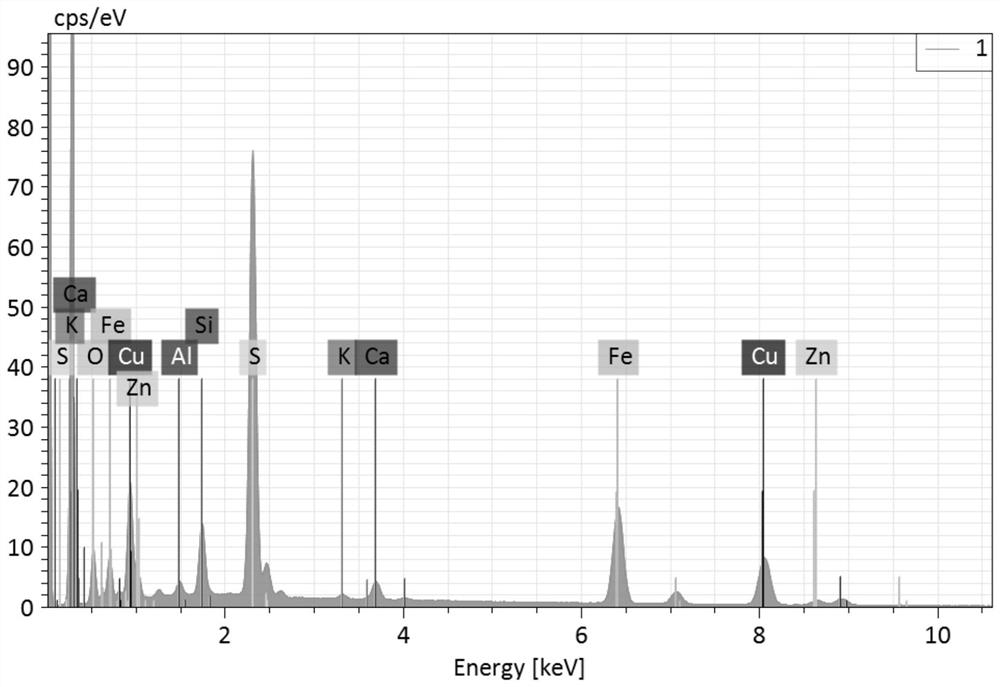
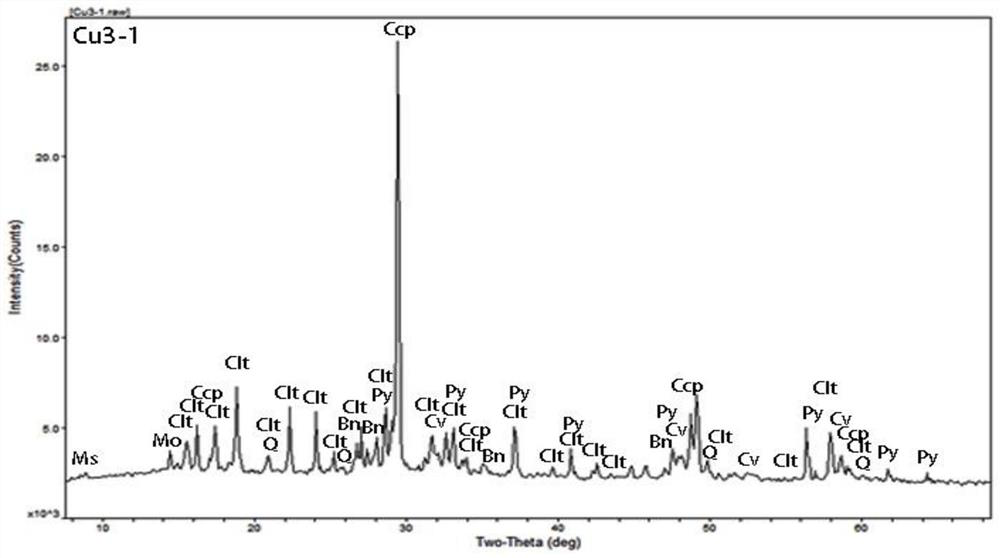
[0060] A certain imported copper concentrate sample is processed according to the experimental process in Example 1 to obtain XRF and XRD patterns such as: figure 2 , image 3 As shown, it can be known that the imported copper concentrate sample species contains bile vitriol (belongs to water-soluble copper), chalcopyrite (belongs to copper oxide), chalcopyrite and bornite (belongs to copper sulfide) phases, and the natural copper content low, not clearly shown in the map.
[0061] The contents of water-soluble copper, copper oxide and natural copper measured by atomic absorption spectrometry are: 6.05% (distributed 25.74% of the total copper content), 0.15% (distributed 0.64%), 0.08% (distributed 0.34%); The copper sulfide content by titration was 17.20% (73.28% allocated) and the total copper content was 23.48%.
[0062] The imported copper concentrate was artificially added with copper sulfate and copper sulfide, and a standard addition recovery experiment was carried ou...
Embodiment 3
[0069] To another batch of imported copper concentrate, handle according to the experimental flow in Example 1, obtain XRF and XRD spectrum such as: Figure 4 , Figure 5 As shown, it can be known that the imported copper concentrate sample species contains chalcopyrite (belonging to copper oxide) and chalcopyrite (belonging to copper sulfide) phases, and the water-soluble copper content is low, which is not clearly displayed in the map.
[0070] The contents of water-soluble copper, copper oxide and natural copper measured by atomic absorption spectrometry are: 0.008% (distribution 0.03%), 0.026% (distribution 0.10%), 0% (distribution 0%); copper sulfide measured by titration The content of 26.30% (99.87% distribution).
[0071] The imported copper concentrate was artificially added with copper sulfate and copper sulfide, and a standard addition recovery experiment was carried out. The recovery rate was 90-110%, which could verify the accuracy of the method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com