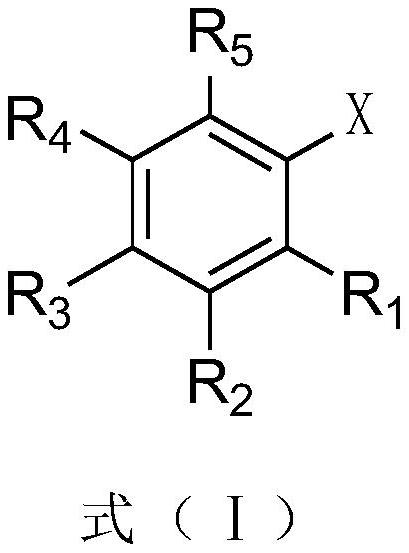
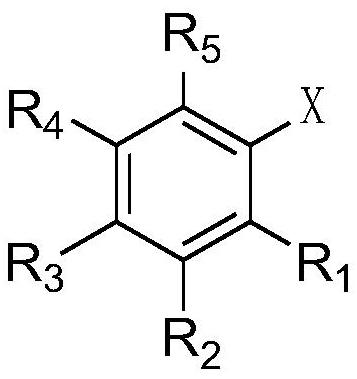
Cesium neopentanoate co-catalyzed aryl borate preparation method
A technology of aryl boronate and cesium pivalate, applied in the field of preparation of aryl borate, can solve the problems of difficult separation and purification, low utilization rate of transition metal catalyst, low yield of boron acylation product, etc. The effect of avoiding dehalogenation side reactions, short reaction time and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Under the protection of nitrogen, 2.33g of 4-bromobiphenyl (10mmol) and 6.35g of bis-valeryl diboron (25mmol) were dissolved in 50mL of 1,4-dioxane, and then 2.57g of cesium pivalate (11mmol) ), 113mg Pd(OAc)2(0.5mmol) and 262mg PPh 3 (1mmol) of 1,4-dioxane (30ml) was added to the above solution, the reaction heat was raised to 90°C, and the reaction was stirred for 3h. The reaction solution was filtered through celite, the filtrate was collected, concentrated under reduced pressure, and then silica gel column chromatography (eluent: petroleum ether volume: ethyl acetate volume = 10:1) to obtain 2.66 g of the product with a yield of 95%. Purity 98.3% (HPLC).
[0032] 1 H NMR (400MHz, CDCl3) δ7.91(d, J=7.2Hz, 2H), 7.63(d, J=6.8Hz, 2H), 7.58(t, J=6.8, 13.6Hz, 3H), 7.30(d ,J=7.2Hz,2H).1.38(s,12H)ESI-MS, 281.2[M+H] +
[0033]
Embodiment 2
[0035] Under nitrogen protection, 1.85g of p-ethylbromobenzene (10mmol) and 6.35g of bis-valeryl diboron (25mmol) were dissolved in 50mL of 1,4-dioxane, and then 2.57g of cesium pivalate ( 11mmol), 113mg Pd(OAc) 2 (0.5mmol) and 262mg PPh 3 (1mmol) of 1,4-dioxane (30ml) was added to the above solution, the reaction heat was raised to 95°C, and the reaction was stirred for 2h. The reaction solution was filtered through celite, the filtrate was collected, concentrated under reduced pressure, and then silica gel column chromatography (eluent: petroleum ether volume: ethyl acetate volume = 10:1) to obtain 2.20 g of the product with a yield of 94.8%. Purity 99.2% (HPLC).
[0036] 1 H NMR (400MHz, CDCl3) δ7.69 (d, J = 8.4Hz, 2H), 7.08 (d, J = 8.4Hz, 2H), 2.54 (q, J = 6.1, 2H), 1.22 (s, 12H) ,1.33(m,3H,CH 3 ).ESI-MS, 233.2[M+H] +
[0037]
Embodiment 3
[0039] Under nitrogen protection, 2.04g p-3,5-dimethylbromobenzene (11mmol) and 6.35g bisvaleryl diboron (25mmol) were dissolved in 55mL dimethyl sulfoxide, and then 2.81g pivalic acid was dissolved Cesium (12mmol), 113mg Pd(OAc) 2 (0.5mmol) and 314.4mg PPh3 (1.2mmol) of dimethyl sulfoxide (25ml) solution was added to the above solution, the reaction heat was raised to 87°C, and the reaction was stirred for 3h. The reaction solution was filtered through diatomaceous earth, the filtrate was collected, concentrated under reduced pressure, and then silica gel column chromatography (eluent: petroleum ether volume: ethyl acetate volume = 10:1) to obtain 2.37 g of the product with a yield of 92.8%. Purity 98.5% (HPLC).
[0040] 1 H NMR (400MHz, CDCl3) δ7.44(s,2H),7.10(s,1H),2.32(s,6H),1.34(s,12H).ESI-MS, 233.2[M+H] +
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com