Fluorine-containing terminal group active hydroxyl friction-resistant high-shear-resistant composite rubber material
A composite rubber and anti-friction technology, which is applied in coatings and other directions, can solve the problems of non-discovered friction-resistant rubber and plastic materials containing fluorine-containing end groups and active hydroxyl groups, and achieve the goals of overcoming interfacial bonding force, improving friction performance, and excellent thermal performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] (1) Preparation and purification of dihalogenated monomers with different soft segment lengths:
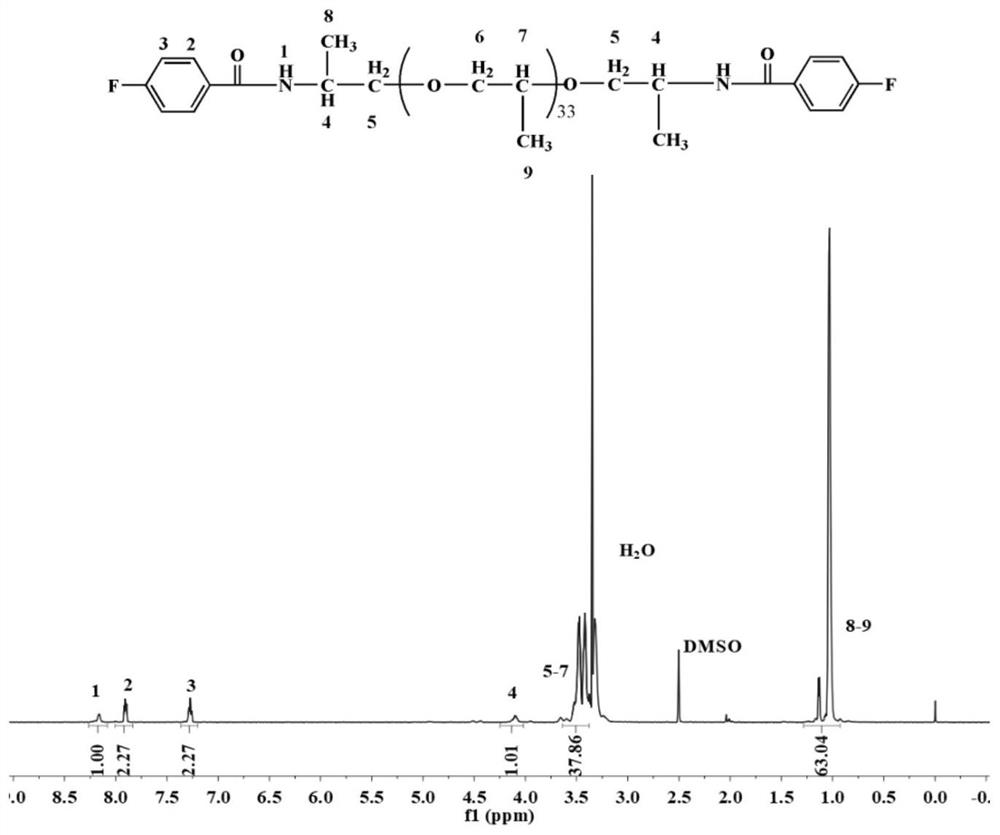
[0080] Add 2000 g of ether-containing diamine containing 33 alkyl carbons, 80 g of sodium hydroxide, and 3 g of sodium dodecylbenzenesulfonate into 5000 g of deionized water in sequence, and dissolve at room temperature to obtain an ether-containing diamine solution; Mix 317g of fluorobenzoyl chloride and 5000g of dichloromethane evenly and add it to the container, then add the ether-containing diamine solution prepared above into the container dropwise, and continue the reaction at room temperature for 5h after the diamine solution is added dropwise , to generate difluoro monomers containing 33 alkylcarbodiamides; the organic solvent in the above monomers was distilled and recovered, and the viscous crude product was collected, washed 5 times with water, solvent removed, and dried at 110°C for 6 hours to obtain purified 33 alkyl carbodiamide difluoro monomers, the structur...
Embodiment 2
[0089] (1) Preparation and purification of dihalogenated monomers with different soft segment lengths:
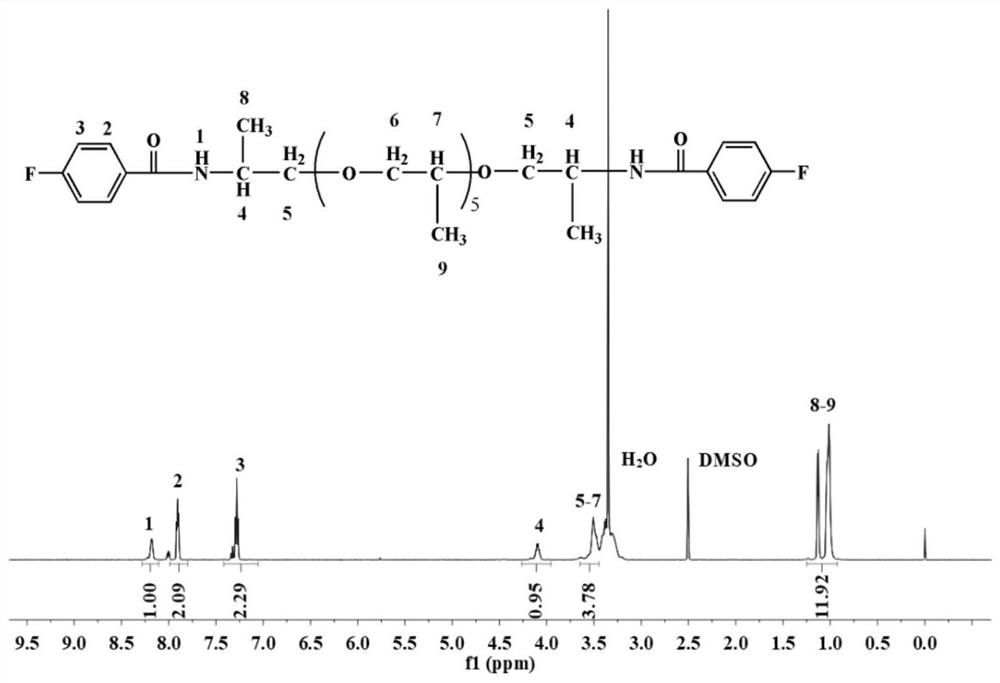
[0090] Add 400g of ether-containing diamine containing 5 alkyl carbons, 90g of lithium hydroxide, and 10g of PEG-1000 into 4000g of deionized water in sequence, and dissolve at room temperature to obtain an ether-containing diamine solution; 317g of p-fluorobenzoyl chloride Mix it with 4000g of chloroform evenly and add it to the container, then drop the prepared diamine solution containing ether into the container. After the diamine solution is added dropwise, continue to react for 3 hours at room temperature to form a Carbodiamide difluoro monomer; distill and recover the organic solvent in the above monomers, collect the viscous crude product, wash with water 4 times, remove the solvent, and dry at 100°C for 24 hours to obtain purified carbodiamide containing 5 alkyl groups Difluoromonomer, structural formula is as follows (NMR such as figure 2 shown):
[0091]
[...
Embodiment 3
[0099] (1) Preparation and purification of dihalogenated monomers with different soft segment lengths:
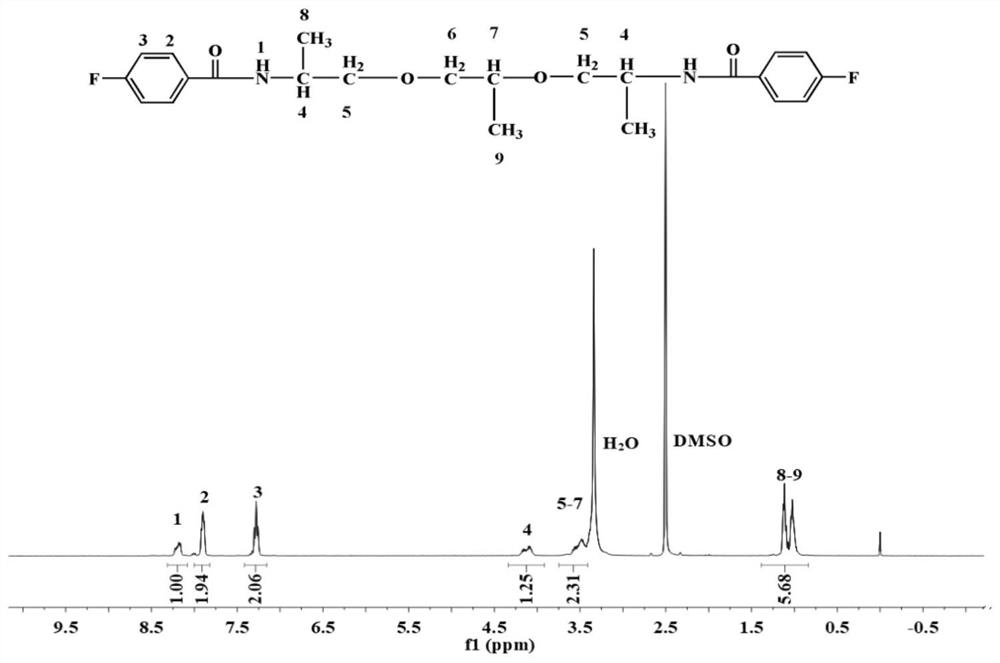
[0100] Add 230 g of ether-containing diamine containing 2 alkyl carbons, 100 g of triethylamine, and 20 g of Tween 80 into 4500 g of deionized water in sequence, and dissolve at room temperature to obtain an ether-containing diamine solution; 317 g of p-fluorobenzoyl chloride Mix it with 3500g of chlorobenzene evenly and add it into the container, then add the above prepared diamine solution containing ether dropwise into the container, after the diamine solution is added dropwise, continue to react for 5 hours at room temperature to generate Carbodiamide dihalogenated monomer; distill and recover the organic solvent in the above monomer, collect the viscous crude product, wash 4 times with water, remove the solvent, and dry at 100°C for 12 hours to obtain purified carbon dihalide containing 2 alkyl Amide difluorinated monomer, the structural formula is as follows (NMR such...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| wear volume | aaaaa | aaaaa |
| wear volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com