Friction-resistant and high-shear-resistant composite rubber material containing fluorine-terminated active hydroxyl groups
A composite rubber and anti-friction technology, which is applied in the field of anti-friction materials, can solve the problems of non-discovered friction-resistant rubber and plastic materials containing fluorine-containing end groups and active hydroxyl groups, and achieve the goal of overcoming interface bonding force, improving friction performance, and improving friction performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
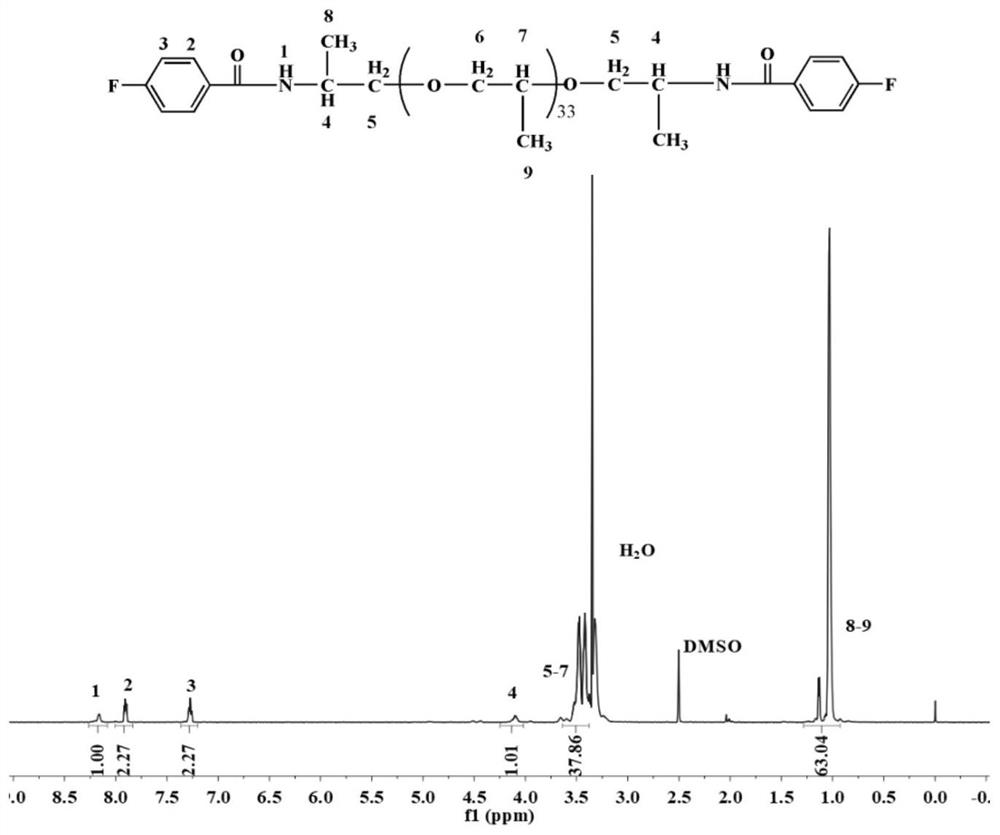
[0079] (1) Preparation and purification of dihalogenated monomers containing different soft segment lengths:
[0080] Add 2000 g of ether-containing diamine containing 33 alkyl carbons, 80 g of sodium hydroxide, and 3 g of sodium dodecyl benzene sulfonate to 5,000 g of deionized water in turn, and dissolve at room temperature to obtain ether-containing diamine solution; 317g of fluorobenzoyl chloride and 5000g of dichloromethane were mixed evenly and added to the container, and then the ether-containing diamine solution prepared above was added dropwise into the container. After the diamine solution was added dropwise, the reaction was continued at room temperature for 5h , to generate 33 alkylcarbodiamide difluorinated monomers; the organic solvent in the above monomers was distilled and recovered, and the viscous crude product was collected, washed with water for 5 times, removed the solvent, and dried at 110 °C for 6 h to obtain a purified 33 alkyl carbodiamide difluoro mon...
Embodiment 2
[0089] (1) Preparation and purification of dihalogenated monomers containing different soft segment lengths:
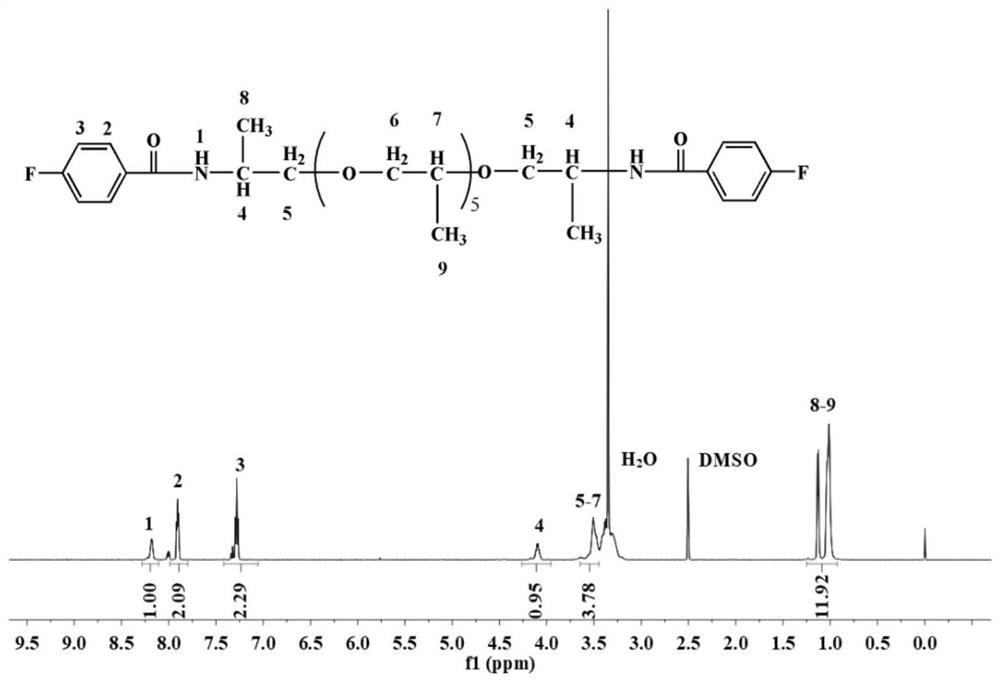
[0090] Add 400 g of ether-containing diamine containing 5 alkyl carbons, 90 g of lithium hydroxide, and 10 g of PEG-1000 to 4000 g of deionized water successively, and dissolve at room temperature to obtain ether-containing diamine solution; 317 g of p-fluorobenzoyl chloride Mix it with 4000g of chloroform and add it to the container, then add the ether-containing diamine solution prepared above dropwise into the container. After the diamine solution is added dropwise, continue to react at room temperature for 3h to generate 5 alkyl groups. Carbodiamide difluorinated monomer; the organic solvent in the above monomer is recovered by distillation, the viscous crude product is collected, washed with water for 4 times, the solvent is removed, and dried at 100°C for 24h to obtain a purified carbodiamide containing 5 alkyl groups Difluorinated monomer, the structural formul...
Embodiment 3
[0099] (1) Preparation and purification of dihalogenated monomers containing different soft segment lengths:
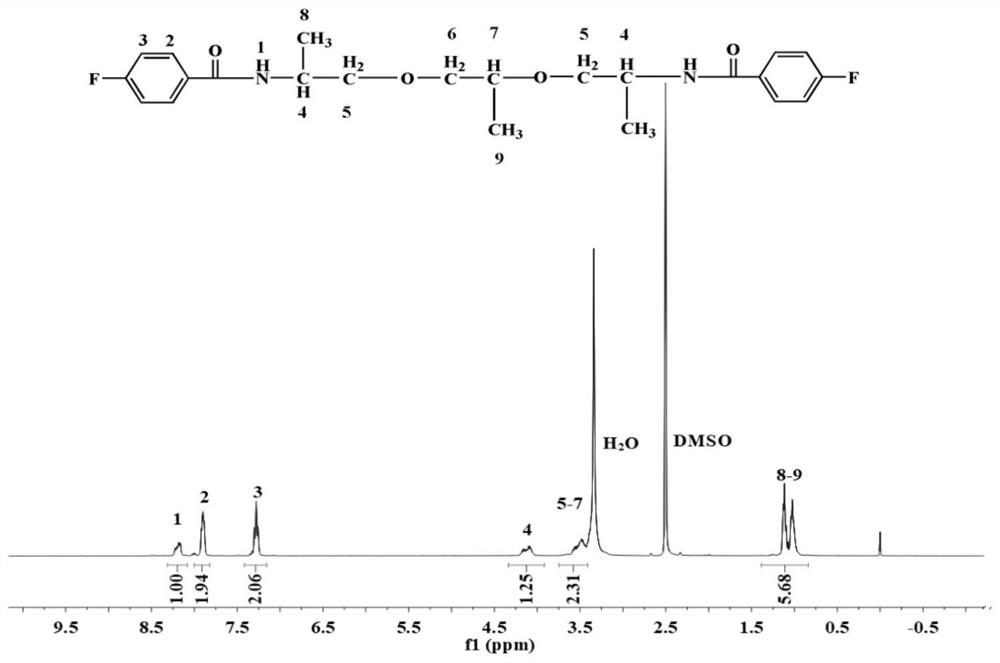
[0100] 230 g of ether-containing diamine containing 2 alkyl carbons, 100 g of triethylamine, and 20 g of Tween 80 were added to 4,500 g of deionized water in turn, and dissolved at room temperature to obtain ether-containing diamine solution; p-fluorobenzoyl chloride 317 g Mix it with 3500g of chlorobenzene and add it to the container. Then add the ether-containing diamine solution prepared above dropwise into the container. After the diamine solution is added dropwise, continue to react for 5h at room temperature to generate 2 alkanes. carbodiamide dihalogenated monomer; the organic solvent in the above monomer is recovered by distillation, the viscous crude product is collected, washed with water for 4 times, the solvent is removed, and dried at 100°C for 12h to obtain a purified product containing 2 alkyl carbodiamides. Amide difluorinated monomer, the structural f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| wear volume | aaaaa | aaaaa |
| wear volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com