Preparation method and application of bombyx mori antibacterial peptide BMGlvA2 recombinant protein
A silkworm antibacterial peptide and recombinant protein technology, applied in the fields of genetic engineering and molecular biology, can solve the problems of restricting industrial production and application, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
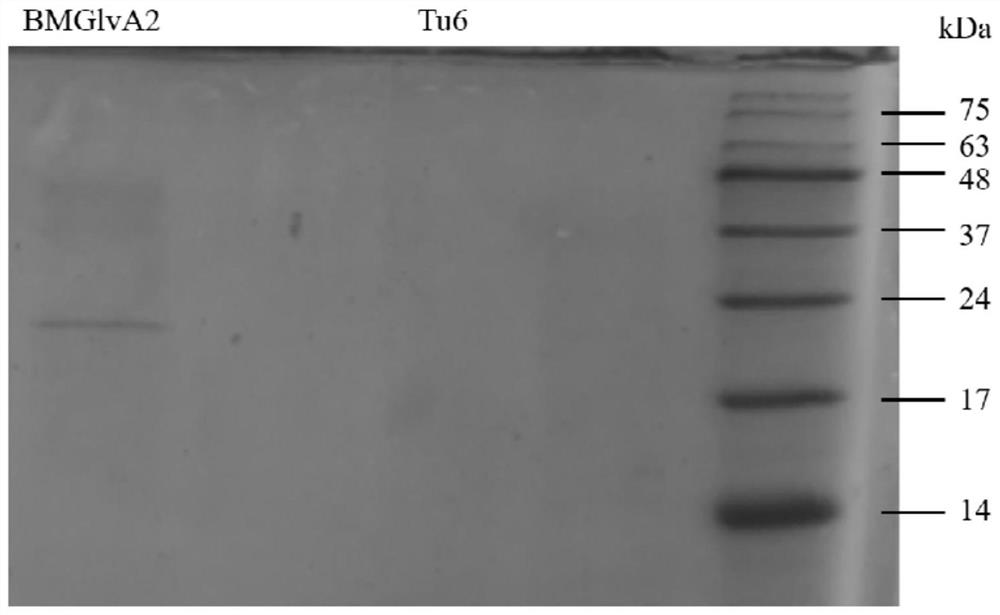
[0068] Construction of antimicrobial peptides BMGlvA2 engineering bacteria
[0069] Determining (1) the amino acid sequence of the antimicrobial peptide BMGlvA2 recombinant protein
[0070] The amino acid sequence silkworm Bombyx mori (domestic silkworm) antimicrobial peptides derived from the amino acid sequence disclosed in GenBank BMGlvA2 No.AB239448 original amino acid sequence, by error prone PCR and screened for the recombinant protein BMGlvA2 antimicrobial peptide, such as SEQ ID NO. 1 shown in FIG.
[0071] (2) Construction of recombinant expression vector
[0072] The recombinant protein synthesis BMGlvA2 antimicrobial peptide sequences using PCR fidelity synthase was amplified by PCR reaction. PCR amplification procedure is 95 ℃ denaturation 5min, 95 ℃ denaturation 30s, 60 deg.] C annealing 30s, extension 30s 72 ℃, 35 cycles, then extended 10min 72 ℃. The amplified fragment Trichoderma expression vector into E. coli DH5α competent cells in vitro recombinant connected, an...
Embodiment 2
[0074] BMGlvA2 antimicrobial peptide expression of recombinant proteins in Trichoderma reesei in
[0075] (1) transforming the recombinant plasmid in Trichoderma reesei
[0076] The T. reesei host strains in solid medium plates PDA + U subcultured conditions were 30 ℃ 5-6 days. The mature T. reesei host strain was inoculated to YEG culture medium, as an expression host 20h at 30 ℃ 180rpm conditions.
[0077] Mature expression host cultured mycelium was collected, and rinsed with sterile water. Weigh the washed mycelia to 1-2g standby 100mL Erlenmeyer flask, which was cleaved under the conditions of 30 ℃ 90rpm, every 30min number of protoplasts prepared samples under a microscope using a hemocytometer calculated until it reaches 10 8 CFU / mL stop cracking. The filtrate was collected after lysis was filtered, centrifuged and washed continuously with sorbitol solution, Trichoderma protoplasts were collected.
[0078] (2) The recombinant expression plasmid flask fermentation of Trich...
Embodiment 3
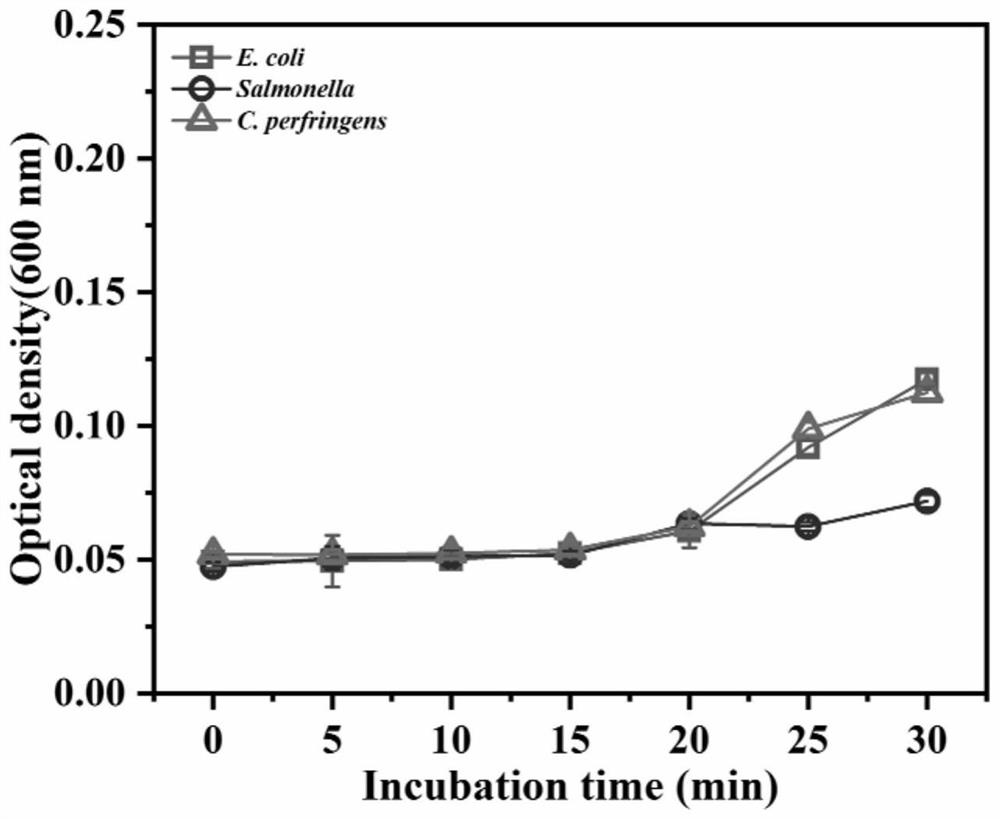
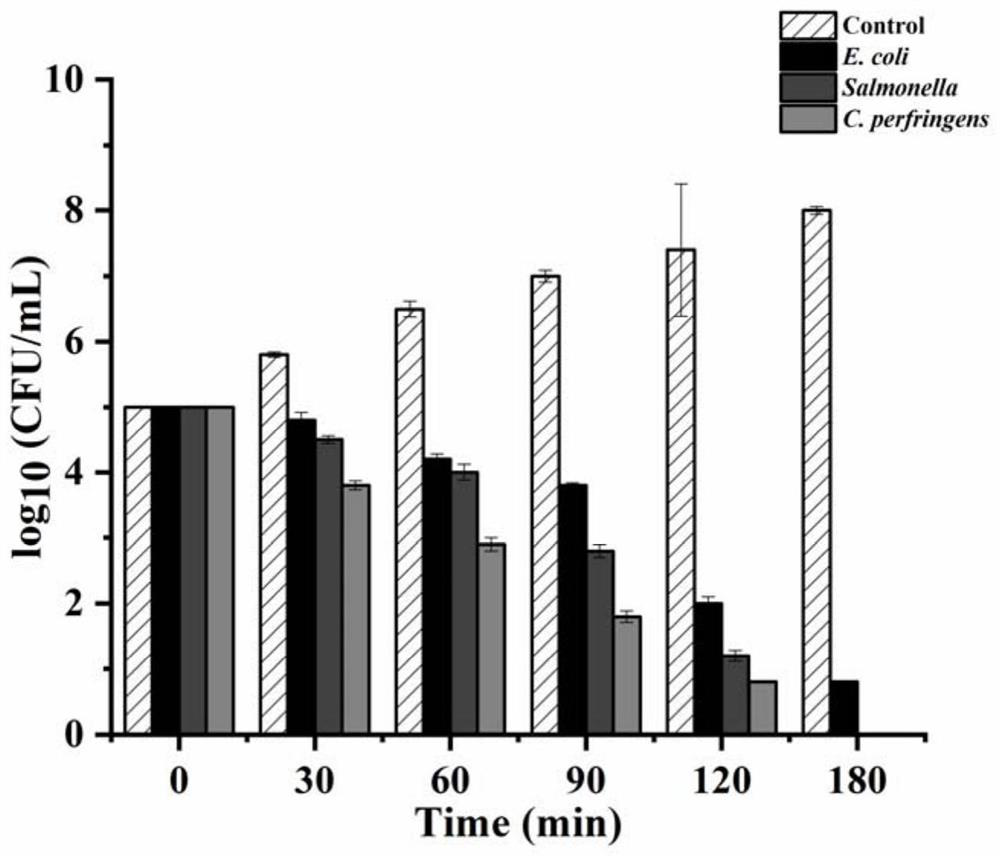
[0082] Detecting the recombinant protein antibacterial activity of antimicrobial peptides of BMGlvA2
[0083] (1) Determination of antibacterial peptide BMGlvA2 recombinant protein in bacteria MIC
[0084] MIC were measured by micro dilution method. Including bacterial suspension was prepared, antimicrobial peptides processing BMGlvA2 recombinant protein detection.
[0085] Preparation of the bacterial suspension: E. coli, Salmonella grown to log phase growth, the same Clostridium perfringens grown to log phase growth in BHI broth in a LB liquid medium. The three kinds of bacteria cells were respectively diluted with the appropriate liquid medium until the concentration was 10 5 CFU / ml.
[0086] Peptide BMGlvA2 processing the recombinant protein: The fermentation product was sterilized by filtration and lyophilized to a powder. Weighed amount of sample powder was reconstituted in sterile water. In each 96-well plate was added 100μL of the appropriate liquid media pathogens, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com