Gene medicine for preventing and treating neovascular eye diseases
A technology for eye diseases and genes, which is applied in the fields of cardiovascular system diseases, gene therapy, sensory diseases, etc., can solve the problems that subjects are prone to infection and inflammation, difficult to apply, etc., to achieve inhibition of proliferation and migration activity, inhibition of proliferation, The effect of inhibiting angiogenesis ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: VEGFA shRNA sequence design and PEDF overexpression adeno-associated virus plasmid construction
[0073] (1) Construction of VEGFA interference vector
[0074] First search the sequence information of human VEGFA (NM_001025366.3) on NCBI, and then use the online design software http: / / rnaidesigner.thermofisher.com / rnaiexpress / design.do to predict the shRNA interference sequence of VEGFA. Add the sequence corresponding to the restriction site XhoI in the middle of the sequence as a hairpin structure, and assemble according to "EcoRI restriction site sticky end-sense strand-XhoI-antisense strand-MluI restriction site sticky end. Synthesize and anneal method, constructing it into plasmid pAAVE2099 (digested by EcoRI and MluI), verified by enzyme digestion, and successfully sequenced, and finally obtained pAAVE2099-shVEGFA-1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 totaled 12 VEGFA interference vectors.
[0075] The transcribed DNA sequences of shVEGFA-1 to 12 a...
Embodiment 2
[0135] Example 2: Packaging of Adeno-Associated Virus Particles
[0136] 5% CO at 37°C 2 293T cells were cultured in a cell incubator, and the medium used was DMEM medium supplemented with 10% fetal bovine serum. Subculture 293T cells in a 10cm-diameter petri dish. After 24 hours (the cells are in the algebraic growth phase), select a petri dish with a cell confluence of about 50%, and replace it with DMEM medium containing 2% fetal bovine serum to culture the cells.
[0137]Prepare the transfection mixture of target plasmids (pAAVE2099, pAAVE2099-shVEGFA-9, pAAVE2099-PEDF, AAV-PEDF-shVEGFA-9), packaging plasmids (pHelper, pAAV-RC2) and liposome transfection reagent. The mixture was added to the cells that had been exchanged for transfection, and the DMEM medium containing 5% fetal calf serum was replaced with fresh DMEM medium after 12 hours. Cells were harvested 72 h after transfection. Add PBS to resuspend the cell pellet, freeze and thaw three times at -80°C / 37°C, centr...
Embodiment 3
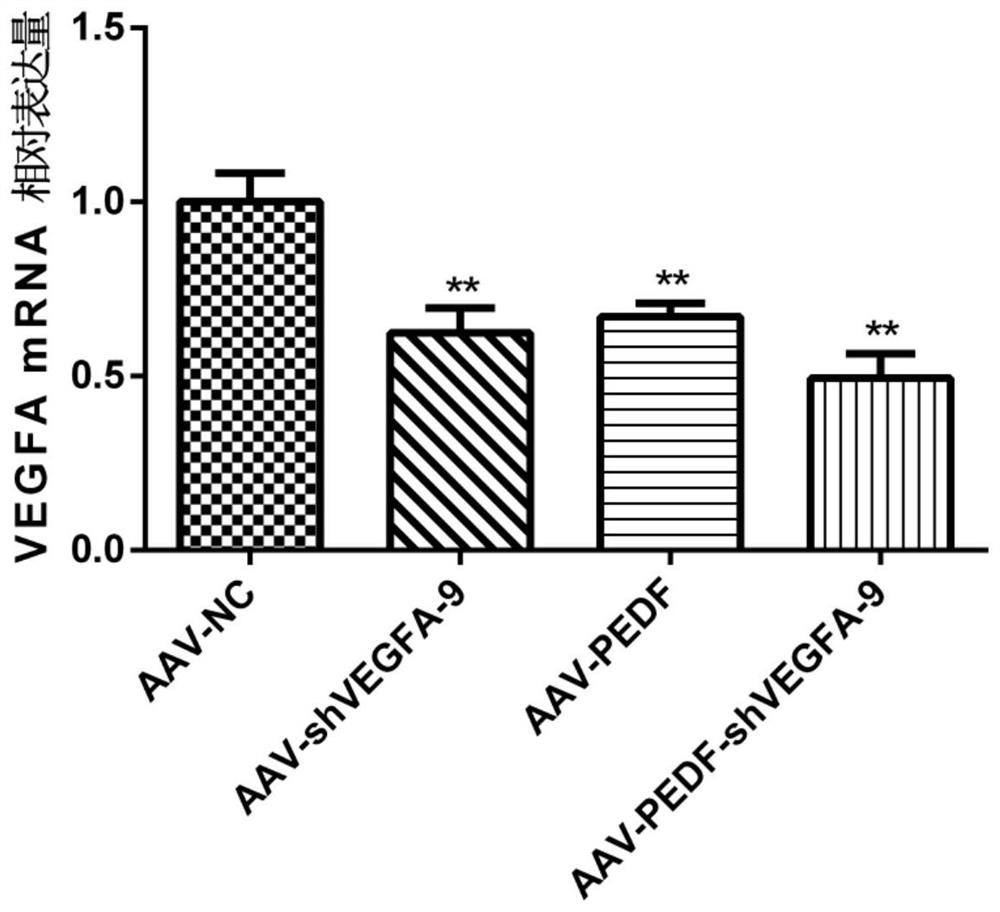
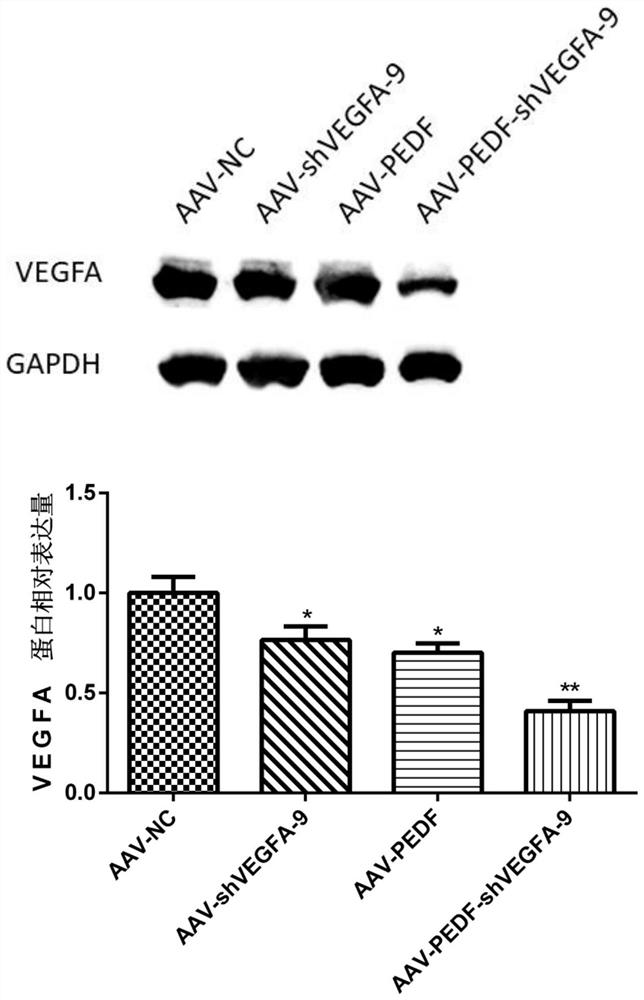
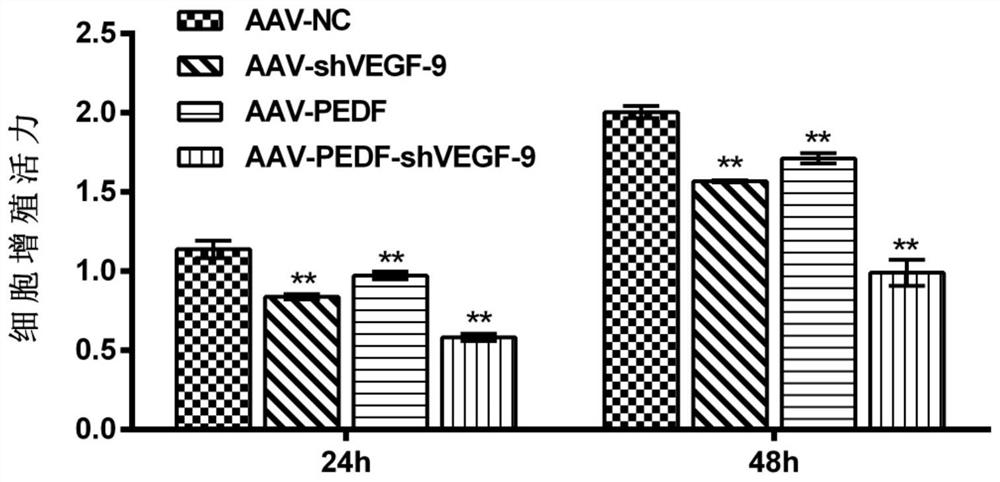
[0139] Example 3: Detection of VEGFA mRNA level
[0140] HUVEC cells were divided into four groups (AAV-NC group, AAV-shVEGFA-9 group, AAV-PEDF group, AAV-PEDF-shVEGFA-9 group). The cells in each group were infected with the corresponding adeno-associated virus (the multiplicity of infection was 10), and cultured for 4 days. Total cellular RNA was extracted by the Trizol method, and RNA was reverse-transcribed into cDNA using random primers. The qPCR reaction system included 5 μL of 2×SYBRGreen Mix, 0.2 μL of upstream and downstream primers (5 pmol / mL), 0.2 μL of cDNA template, ddH 2 O supplemented to 10 μL. The reaction program was [95°C 2min; (95°C 15s, 60°C 30s, 72°C 30s)×40 cycles; melting curve]. With GAPDH as internal reference, use 2 -△△CT Calculate the relative expression level. The primer sequences involved in the experiment are as follows:
[0141] VEGF-F: 5'-AGGGCAGAATCATCACGAAGT-3' (SEQ ID NO. 33);
[0142] VEGF-R: 5'-AGGGTCTCGATTGGATGGCA-3' (SEQ ID NO. 34);...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com