Sinomenine derivative metabolite as well as preparation method, pharmaceutical composition and application thereof
A technology of metabolites and derivatives, applied in the field of biomedicine, can solve problems such as unsatisfactory treatment effects, adverse reactions of patients, and high prices, and achieve the effects of novel structure, recovery of bone marrow hematopoietic function, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
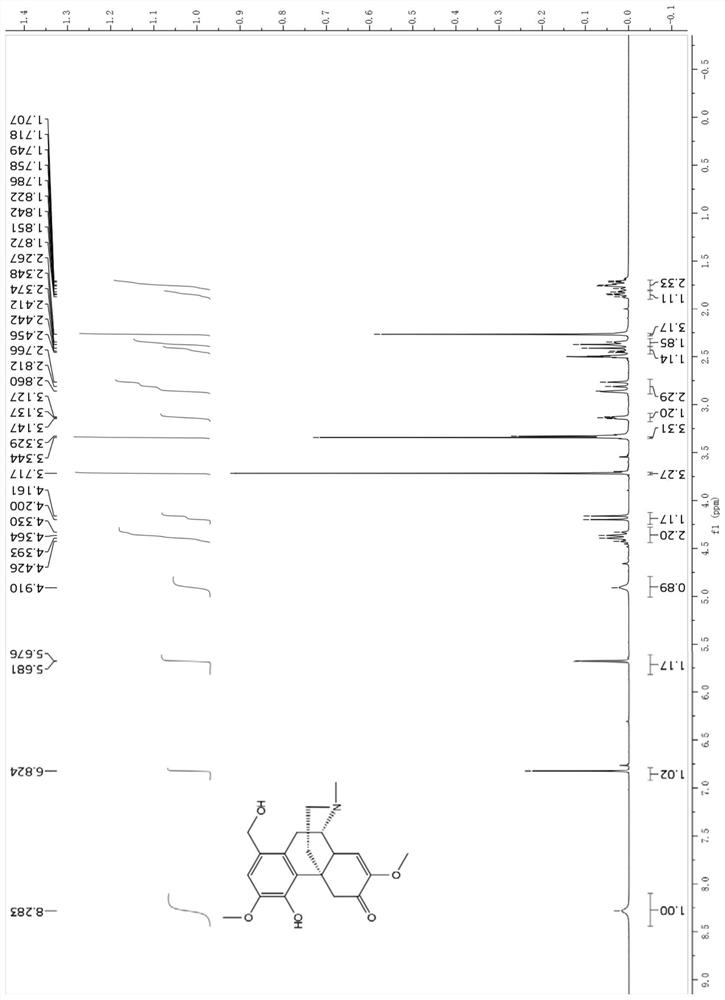
[0047] Example 1: Biosynthesis of sino-wcj-43-M1
[0048] Step 1: using esterase to catalyze the hydrolysis of sino-wcj-43 to sino-wcj-43-M1. The reaction system is PBS buffer (pH=7.4), pig liver esterase 25U / mL, sino-wcj-43 0.5mg / mL, reacted in 37°C water bath for 2h, and the conversion rate of hydrolysis reaction can reach 90% by HPLC-UV detection above.
[0049] Step 2: Add an equal volume of acetonitrile to the reaction solution to precipitate protein, centrifuge at 5500rpm for 10min to remove the protein precipitate, evaporate the supernatant to remove the organic solvent under reduced pressure, extract 5 times with ethyl acetate, evaporate to dryness under reduced pressure, dissolve a small amount of mobile phase, and use the preparation solution Phase separation and purification of sino-wcj-43-M1. The preparative column was Reprosil-Pur Basic C18 (250×10mm, 5 μm, Beim Brueckle14-D-72119Ammerbuch, Germany), and the mobile phase was 27% water (0.02% diethylamine)-73% ac...
Embodiment 2
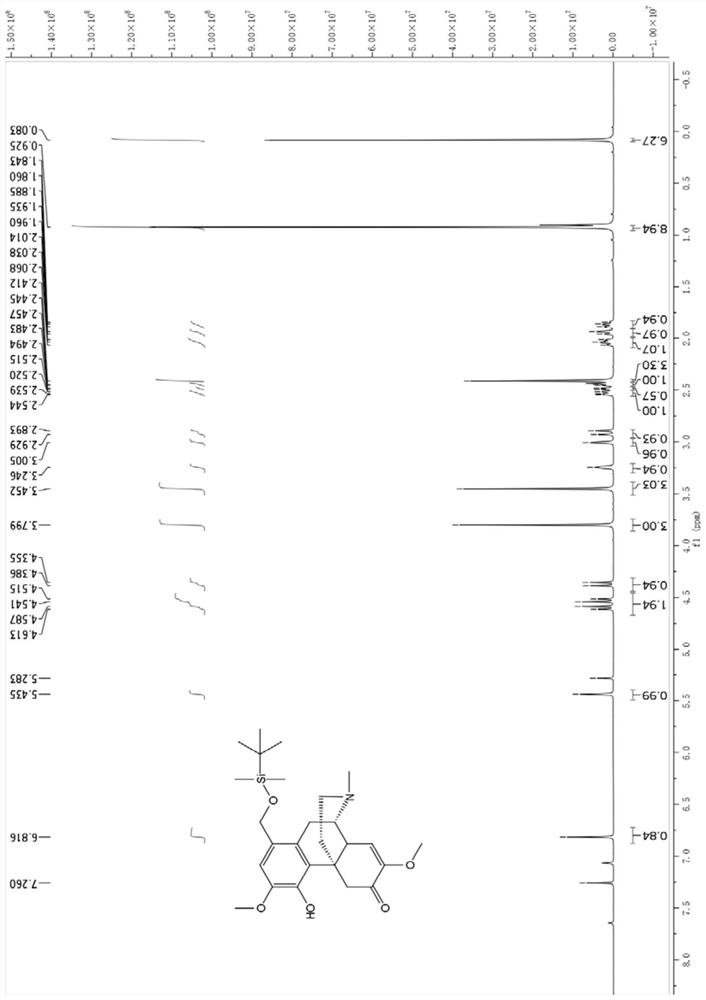
[0051] Embodiment 2: the chemical synthesis of sino-wcj-43-M1
[0052] Molecular formula: C 29 h 31 NO 6
[0053] Name: 1-hydroxymethylene-4-cinnamyloxy-7,8-didehydro-3,7-dimethoxy-17-methyl-morphinan-6-one, 1-hydroxymethylene-4-cinnamyloxy-7,8- Didehydro-3,7-dimethoxy-17-methyl-morphinan-6-one.
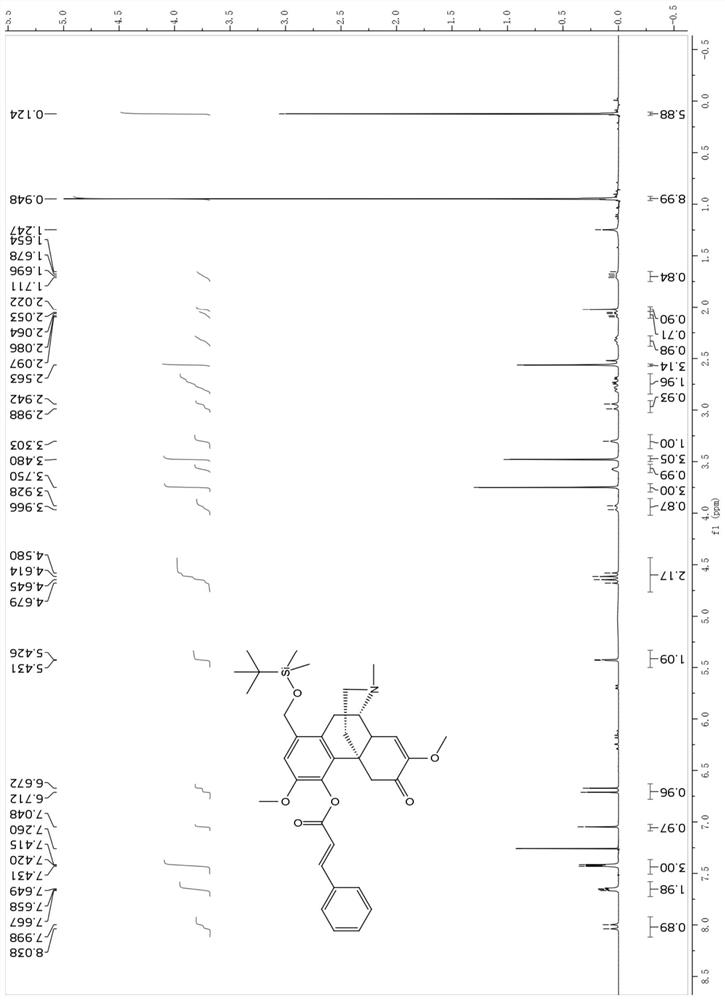
[0054] Using sinomenine hydrochloride as a raw material, heat and reflux with paraformaldehyde to generate 1-hydroxymethylene sinomenine shown in formula 1; 1-hydroxymethylene sinomenine and tert-butyldimethylchlorosilane, Imidazole and 4-dimethylaminopyridine were reacted to obtain intermediate 2; compound 2 was reacted with cinnamic acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 4-dimethyl The reaction of aminopyridine gives intermediate 3; the reaction of compound 3 with hydrogen fluoride and pyridine gives the target compound sino-wcj-43-M1.
[0055]
[0056] Step 1: Weigh 10.0 g of sinomenine hydrochloride, dissolve it in 200 ml of water, add 20.0...
experiment example 1
[0062] Experimental method: 5 male SD rats in each group, administered orally at 50 mg / kg, fasted for 12 hours before the experiment, and had free access to water. At 10, 30 min, 1, 2, 4, 6, 8, 12, and 24 h after administration, blood was collected continuously from the inner canthus venous plexus, anticoagulated with heparin, centrifuged to separate plasma, acetonitrile precipitated protein, and HPLC was used to determine sino-wcj in plasma -43 and sino-wcj-43-M1 content. The measured experimental data application WinNonlin 6.3 software to calculate the pharmacokinetic parameters.
[0063] Experimental results: After intragastric administration of sino-wcj-43 to rats, the prototype drug was not detected in the plasma within the quantitative range of 100-5000ng / ml, but the metabolite sino-wcj-43-M1 could be detected. At the peak, the average peak plasma concentration is 2626ng / ml, AUC 0-t 10281h*ng / ml ( figure 1 ). The experimental results show that after the drug is admin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com