Active photosensitizer and preparation method and application thereof
A photosensitizer and active technology, applied in the field of new drug development, can solve the problems of off-target effect and drug resistance of small molecule inhibitors, and achieve the effect of improving oxidation state and avoiding drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment relates to a kind of quinoxalinone derivative, and its preparation method is as follows: O-phenylenediamine (0.1mol, 10.8g) is dispersed in absolute ethanol (150mL), and ethyl pyruvate (0.12 mol, 13.92g), stirred at room temperature for 12h, the reaction solution was filtered, the filter cake was washed with absolute ethanol, and dried to obtain a white powder 1, (13.6g, yield 86%); 1a (20mmol, 3.2g), K 2 CO 3 (24mmol, 3.31g) was dispersed in acetone, and then bromopropene (24mmol, 3.67g) was added dropwise under stirring, the reaction mixture was reacted overnight at 62°C, the solvent was evaporated to dryness, the residue was added to water and ethyl acetate, separated The ethyl acetate phase was separated on a silica gel column (petroleum ether: ethyl acetate = 10:1), and purified to obtain 3.0 g of compound 2 with a yield of 54%. Compound 2 (2mmol, 500mg) was suspended in acetic acid, thiophene-2-carbaldehyde (3mmol, 570mg) and a catalytic amount of...
Embodiment 2
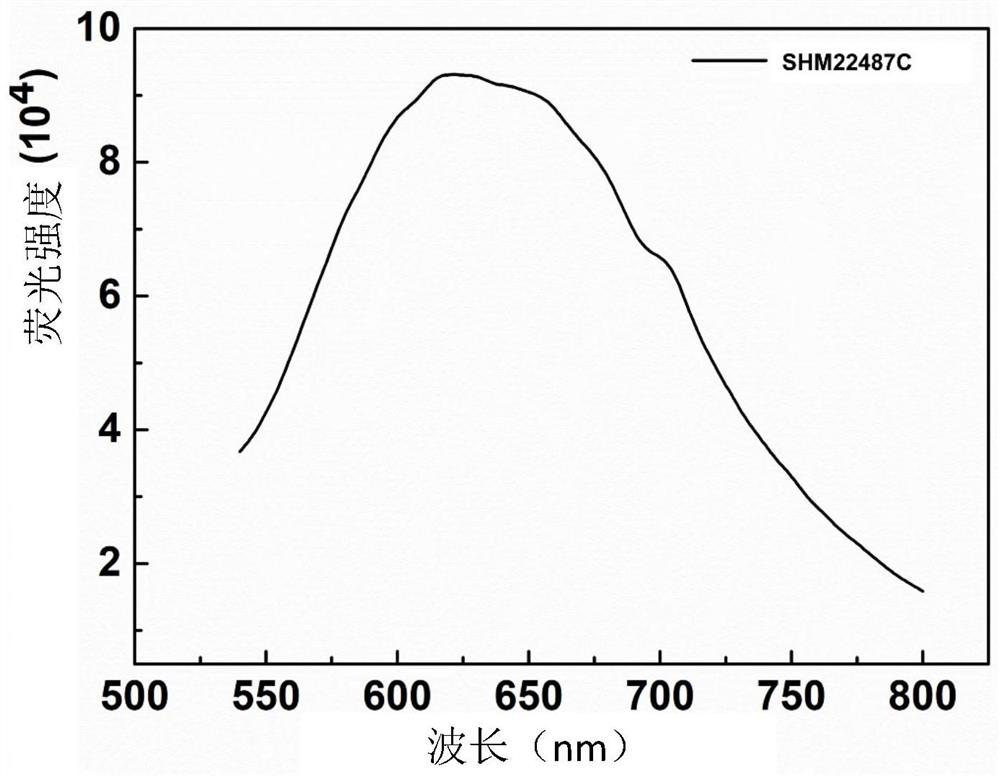
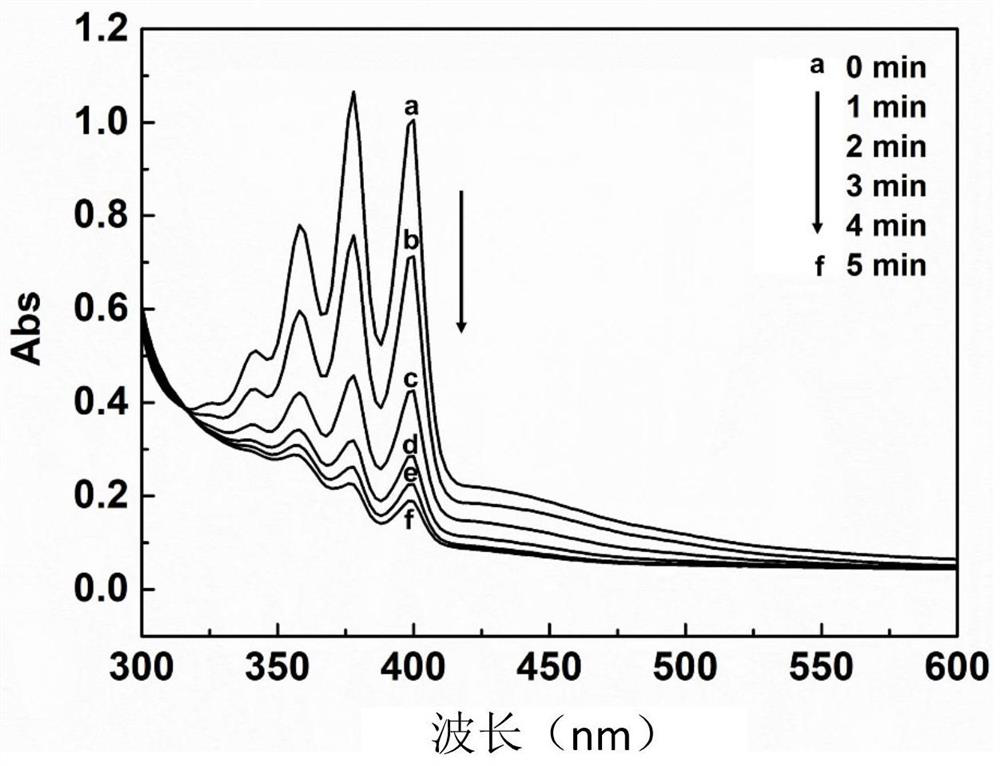
[0040] Configure 1mg / mL SHM22487C DMSO stock solution, and then pass through different ratios of DMSO / H 2 The mixed solvent of O dilutes SHM22487C into a solution with a concentration of 5 μg / mL, and its absorption spectrum is measured by a Thermo Electron-EV300 UV-Vis spectrophotometer. The maximum absorption wavelength of SHM22487C is located at 440nm. Then, the fluorescence spectrum of SHM22487C was measured by a steady-state time-resolved fluorescence spectrophotometer, and it was found that the maximum emission wavelength of SHM22487C reached 610nm. Then, the active oxygen generation efficiency was measured by the active oxygen probe ABDA by UV-Vis spectrophotometer under light. The experimental results showed that SHM22487C had a high active oxygen generation efficiency.
Embodiment 3
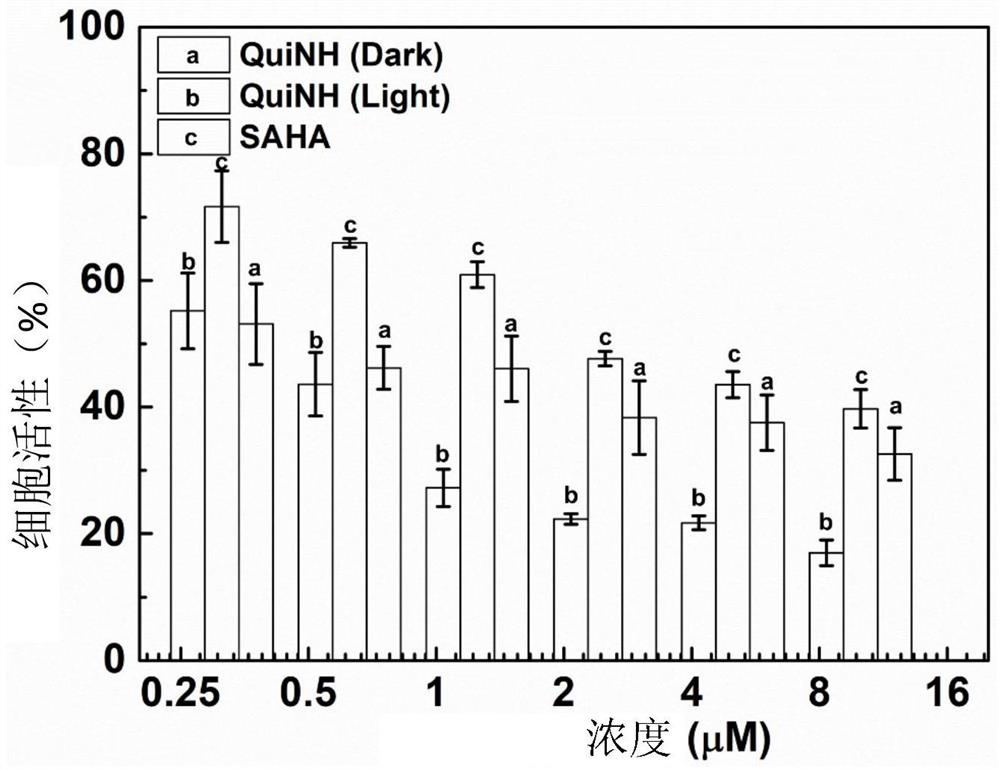
[0042] Prepare a DMSO stock solution containing 1 mg / mL SHM22487C and store it at room temperature in the dark. The human breast cancer cell MCF-7 was planted in a culture dish at a density of 105 / mL. After it adhered to the wall, different concentrations of SHM22487C (0.625 μM, 1.25 μM, 2.5 μM, 5 μM, 10 μM, 20 μM) were added and cultured for 6 hours. , followed by 450nm laser irradiation for 5min and continued culturing for 48h, then added 20μL MTT (5mg / mL), incubated in a 37°C incubator for 4h, removed the medium, added 150μL of dimethyl sulfoxide, and then passed through a microplate reader at 570nm Check the absorbance value.
[0043] Compound ID IC 50 on HDAC1(nM)
[0044] Table 1 shows the results of SHM22487C enzyme activity inhibition research
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com