Synthesis and application of novel pyridinotriazole receptor and luminescent polymer thereof
A technology of polymers and compounds, applied in the field of materials, to achieve good application prospects, simple synthetic routes, and high yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1, polymer PPT-TT-PT-2FBT, R is 5-decyl pentadecyl (formula I-1)
[0089]
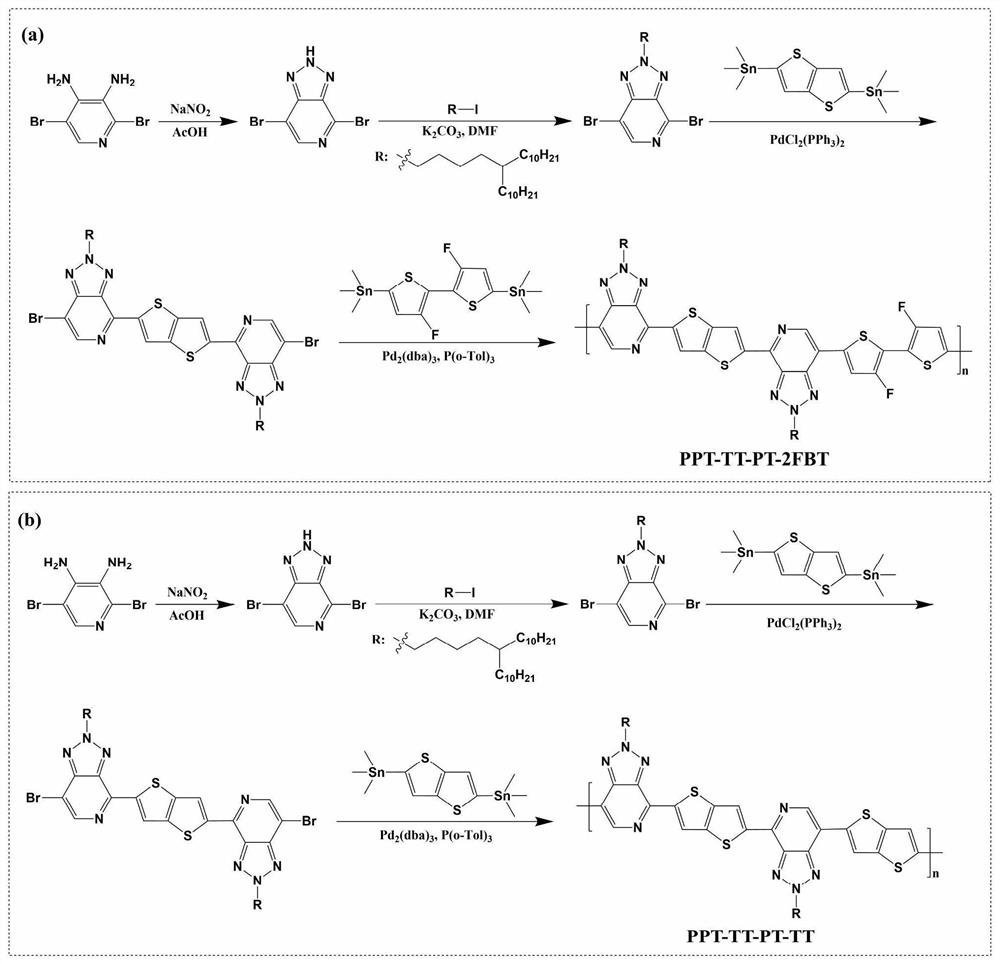
[0090] The reaction equation is as figure 1 (a) shown.
[0091] (1) 4,7-Dibromo-2H-[1,2,3]triazolo[4,5-c]pyridine
[0092] Add 2,5-dibromo-3,4-diaminopyridine (4.00g, 14.99mmol) and 60mL acetic acid into a 100mL two-necked flask, ultrasonicate for 10min, then dissolve sodium nitrite ( 1.55g, 22.48mmol) was dropped into a two-necked flask, and reacted at 30°C for 24h. Wash the product twice with distilled water, then filter the product and transfer it to a 250mL round bottom flask, add about 100mL of ethanol and spin dry (take away water with ethanol), and dry to obtain 4.05g of white solid. Yield: 97.12%.
[0093] The structural characterization data are as follows:
[0094] Mass Spectrum: ESI-MS: [M] - calcd for C 5 HBr 2 N 4 - :276.85,found:276.90.
[0095] H NMR and C NMR: 1 H NMR (400MHz, DMSO) δ8.49 (m, 1H). 13 C NMR (100MHz, DMSO) δ144.65, 140.37, 132.44, 131.32,...
Embodiment 2
[0113] Embodiment 2, polymer PPT-TT-PT-TT, R is 5-decyl pentadecyl (formula I-2)
[0114]
[0115] Wherein the definition of R is the same as the definition of R in the aforementioned formula 1.
[0116] The reaction equation is as figure 1 (b) shown.
[0117] The first three steps are the same polymer as PPT-TT-PT-2FBT;
[0118] Polymer PPT-TT-PT-TT:
[0119] 2,5-bis(7-bromo-2-(5-decylpentadecyl)-2H-[1,2,3]triazolo[4,5-c]pyridine-4 shown in formula 1 -yl)thieno[3,2-b]thiophene (100.0mg, 0.081mmol), 2,5-bis(trimethyltin)thieno[3,2-b]thiophene (37.73mg, 0.081mmol), Catalyst tris(dibenzylideneacetone)dipalladium (2.25mg, 0.0024mmol), ligand tris(o-tolyl)phosphine (5.84mg, 0.019mmol) and chlorobenzene (5mL) were added in the reaction flask, under argon Three freeze-pump-thaw cycles were performed to remove oxygen, and then the reaction mixture was heated to 125° C. for 48 h of polymerization. After cooling, 100 mL of methanol was added, stirred at room temperature for 3 ...
Embodiment 3
[0126] Optical performance, electrochemical performance and field effect transistor performance of embodiment 3, polymer PPT-TT-PT-2FBT and PPT-TT-PT-TT
[0127] (1) Optical and electrochemical properties of polymers PPT-TT-PT-2FBT and PPT-TT-PT-TT
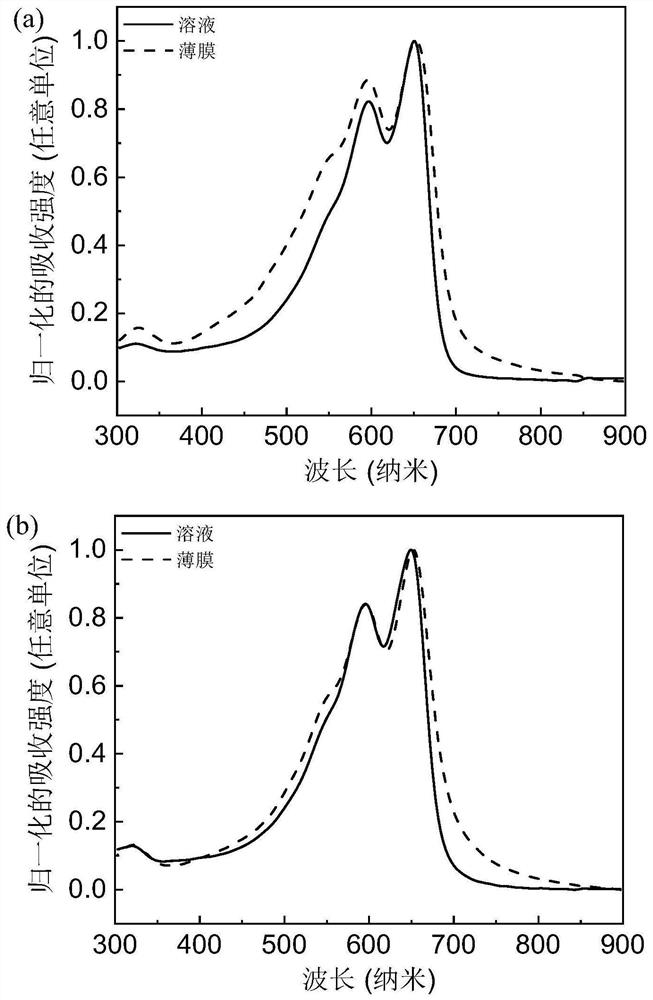
[0128] figure 2 For the polymer PPT-TT-PT-2FBT ( figure 2 (a)) and PPT-TT-PT-TT ( figure 2 (b) UV-Vis absorption spectra in solution and film.
[0129] Depend on figure 2 It can be seen that the polymer film PPT-TT-PT-2FBT ( figure 2 (a)) and PPT-TT-PT-TT ( figure 2 The optical bandgaps of (b)) are 1.77eV and 1.73eV respectively (the optical bandgaps are based on the formula E g =1240 / λ calculation, where E g is the optical band gap, and λ is the boundary value of the UV absorption curve). Depend on figure 2 It can be seen that all three polymers have relatively strong intramolecular charge transfer peaks, indicating that the intermolecular forces of the polymers are relatively strong.
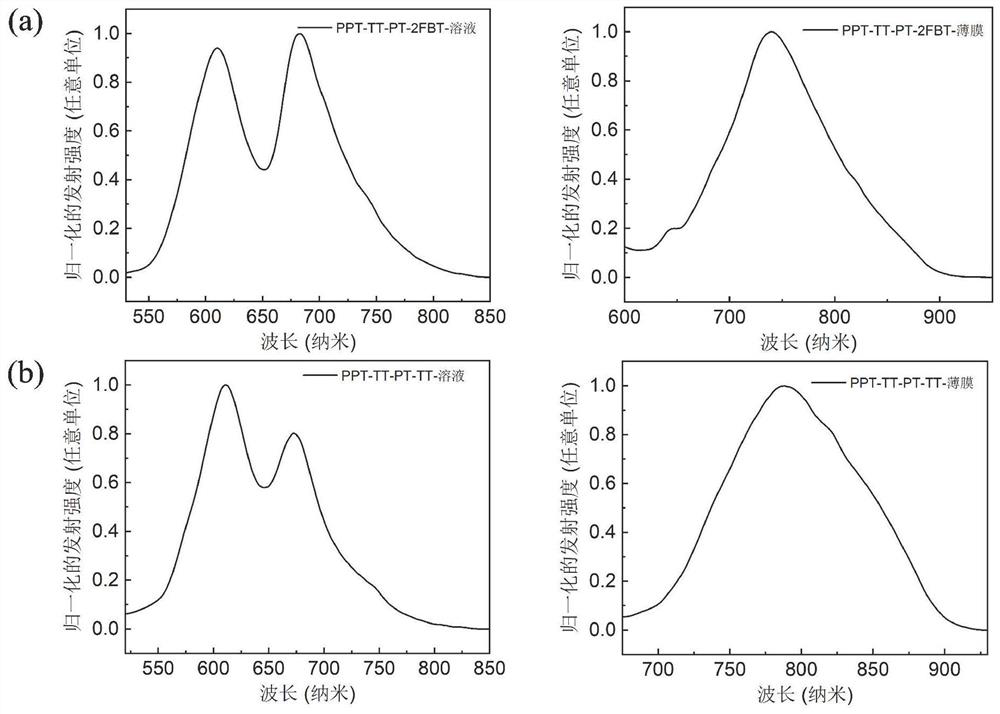
[0130] image 3 For the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical bandgap | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com