Preparation method and application of carbon-nitrogen co-doped iron-cobalt-based catalyst
A co-doping, iron-cobalt-based technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc. problem, to achieve the effect of improving catalytic activity and directional selectivity, increasing active sites, and increasing force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
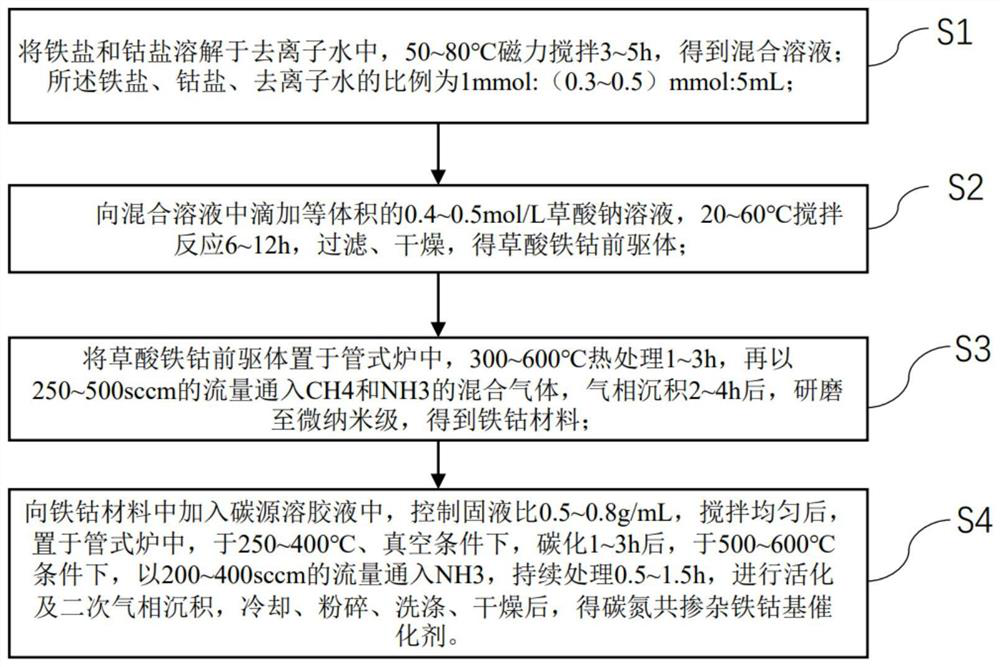
[0025] The present invention provides the preparation method of the carbon-nitrogen co-doped iron-cobalt-based catalyst of an embodiment, comprising the following steps:
[0026] S1: Dissolve iron salt and cobalt salt in deionized water, stir magnetically at 50-80°C for 3-5 hours to obtain a mixed solution; the ratio of iron salt, cobalt salt and deionized water is 1mmol:(0.3-0.5)mmol : 5mL; Described iron salt is one or more in ferrous nitrate, ferrous sulfate, ferrous chloride; Described cobalt salt is one or more in cobalt nitrate, cobalt sulfate, cobalt chloride;
[0027] S2: Add an equal volume of 0.4-0.5mol / L sodium oxalate solution dropwise to the mixed solution, stir and react at 20-60°C for 6-12h, filter, and dry to obtain an iron-cobalt oxalate precursor;
[0028] S3: Place the iron-cobalt oxalate precursor in a tube furnace, heat-treat at 300-600°C for 1-3 hours, and then feed CH at a flow rate of 250-500 sccm 4 and NH 3 mixed gas, after vapor deposition for 2 to ...
Embodiment 1
[0032] A preparation method for a carbon-nitrogen co-doped iron-cobalt-based catalyst, comprising the following steps:
[0033] S1: Dissolve 10 mmol of ferrous nitrate and 3 mmol of cobalt nitrate in 50 mL of deionized water, and stir magnetically at 50° C. for 3 h to obtain a mixed solution;
[0034] S2: Add 50mL of 0.4mol / L sodium oxalate solution dropwise to the mixed solution, stir and react at 20°C for 6h, filter and dry to obtain the iron-cobalt oxalate precursor;
[0035] S3: Place the iron-cobalt oxalate precursor in a tube furnace, heat-treat at 300 °C for 1 h, and then feed CH at a flow rate of 250 sccm 4 and NH 3 the mixed gas, the CH 4 , NH 3 The volume ratio is 1:0.5, and after vapor deposition for 2 hours, it is ground to the micro-nano level to obtain the iron-cobalt material;
[0036] S4: Add 1mL carbon source sol solution to 0.5g iron-cobalt material, and described carbon source sol solution is formulated according to the ratio of 1g:1.5g:10mL by chitosan,...
Embodiment 2
[0038] A preparation method for a carbon-nitrogen co-doped iron-cobalt-based catalyst, comprising the following steps:
[0039] S1: Dissolve 10 mmol of ferrous sulfate and 4 mmol of cobalt sulfate in 50 mL of deionized water, and stir magnetically at 70° C. for 3 to 5 hours to obtain a mixed solution;
[0040] S2: Add 50mL of 0.45mol / L sodium oxalate solution dropwise to the mixed solution, stir and react at 50°C for 8h, filter and dry to obtain the iron-cobalt oxalate precursor;
[0041] S3: Place the iron-cobalt oxalate precursor in a tube furnace, heat-treat at 450°C for 1-3h, and then feed CH at a flow rate of 350sccm 4 and NH 3 the mixed gas, the CH 4 , NH 3 The volume ratio is 1:0.6, and after vapor deposition for 2 to 4 hours, it is ground to the micro-nano level to obtain iron-cobalt materials;
[0042] S4: Add 1mL carbon source sol solution to 0.6g iron-cobalt material, described carbon source sol solution is formulated according to the ratio of 1g:2g:10mL by chit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com