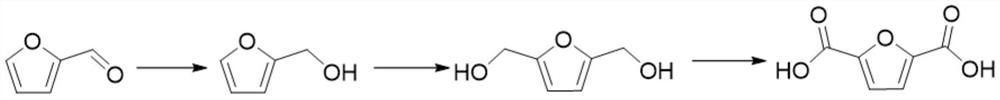
Method for preparing 2, 5-furandicarboxylic acid from furfural
A technology for furandicarboxylic acid and furandicarboxylate is applied in the field of preparation of biomass-based furfural to synthesize 2,5-furandicarboxylic acid, and can solve the problems of complex process, high reaction temperature, harsh reaction conditions and the like, and achieve high product purity, Simple production process and wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Selective hydrogenation of furfural to furfuryl alcohol
[0025] Hydrogenation catalysts are prepared by impregnation. A certain amount of PdCl 2 Dissolved in an appropriate amount of water, weighed 1 g SiO 2 Add to PdCl 2 In the solution, the loading amount of metal Pd is 2%. Stir the mixture until dry, dry it overnight at 80°C, and grind it into powder. The powder is calcined at 350°C in a nitrogen atmosphere, then reduced with hydrogen at the same temperature, cooled and then passed through a mixture of nitrogen and air for aging to obtain a hydrogenation catalyst.
[0026] The present embodiment takes furfural as raw material. Add 0.2g of hydrogenation catalyst into the reactor, add 1g of furfural, and then add 40mL of anhydrous methanol as a solvent. After sealing the reactor, first use N 2 Replace the air in the reactor, and then use H 2 Replace the N in the reactor 2 , then filled with 2MPa of H 2 . The reaction kettle was heated to 150°C and react...
Embodiment 2
[0034] (1) Selective hydrogenation of furfural to furfuryl alcohol
[0035] Hydrogenation catalysts are prepared by impregnation. Dissolve a certain amount of nickel nitrate in an appropriate amount of water, add 1g of ZrO to the solution 2 , so that the loading of metal Ni is 10%. Stir the mixture until dry, dry it overnight at 80°C, and grind it into powder. The powder is calcined at 450°C in a nitrogen atmosphere, then reduced with hydrogen at the same temperature, cooled and then passed through a mixture of nitrogen and air for aging to obtain a hydrogenation catalyst.
[0036] The present embodiment takes furfural as raw material. Add 1g of hydrogenation catalyst into the reactor, add 10g of furfural, and then add 40mL of n-hexane as a solvent. After sealing the reactor, first use N 2 Replace the air in the reactor, and then use H 2 Replace the N in the reactor 2 , then filled with 4MPa of H 2 . The reaction kettle was heated to 180°C and reacted for 2 hours.
...
Embodiment 3
[0044] (1) Selective hydrogenation of furfural to furfuryl alcohol
[0045] Hydrogenation catalysts are prepared by impregnation. Dissolve a certain amount of ruthenium chloride into an appropriate amount of water, add 1 g of activated carbon to the solution, and keep the metal Ru loading at 1.5%. Stir the mixture until dry, dry it overnight at 80°C, and grind it into powder. The powder is calcined at 350°C in a nitrogen atmosphere, then reduced with hydrogen at the same temperature, cooled and then passed through a mixture of nitrogen and air for aging to obtain a hydrogenation catalyst.
[0046] The present embodiment takes furfural as raw material. Add 0.2g of hydrogenation catalyst into the reactor, add 2g of furfural, and then add 40mL of tetrahydrofuran as a solvent. After sealing the reactor, first use N 2 Replace the air in the reactor, and then use H 2 Replace the N in the reactor 2 , then filled with 5MPa of H 2 . The reaction kettle was heated to 140°C and r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com