Construction method and application of mutant of fusion enzyme capable of simultaneously degrading aflatoxin B1 and zearalenone
A technology for zearalenone and aflatoxin, applied in the fields of biotechnology and genetic engineering, can solve the problems of low efficiency, complicated experiments, poor integrity of detoxification effect, etc., and achieve the effect of improving the degradation rate and high degradation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The selection and mutation method of embodiment 1 fusion enzyme mutant mutation site
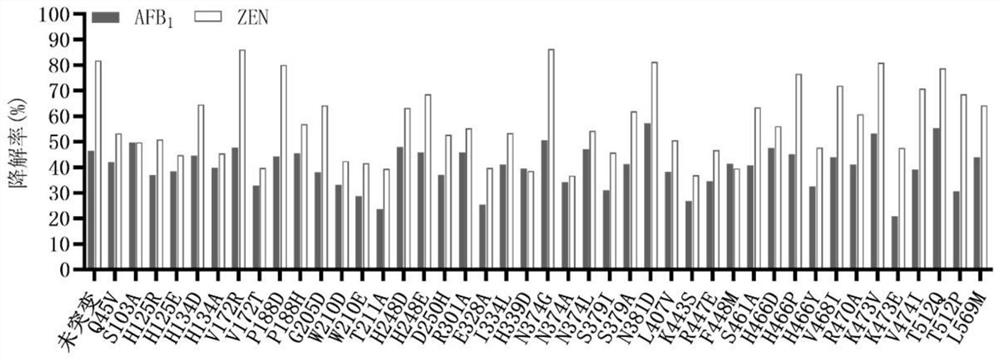
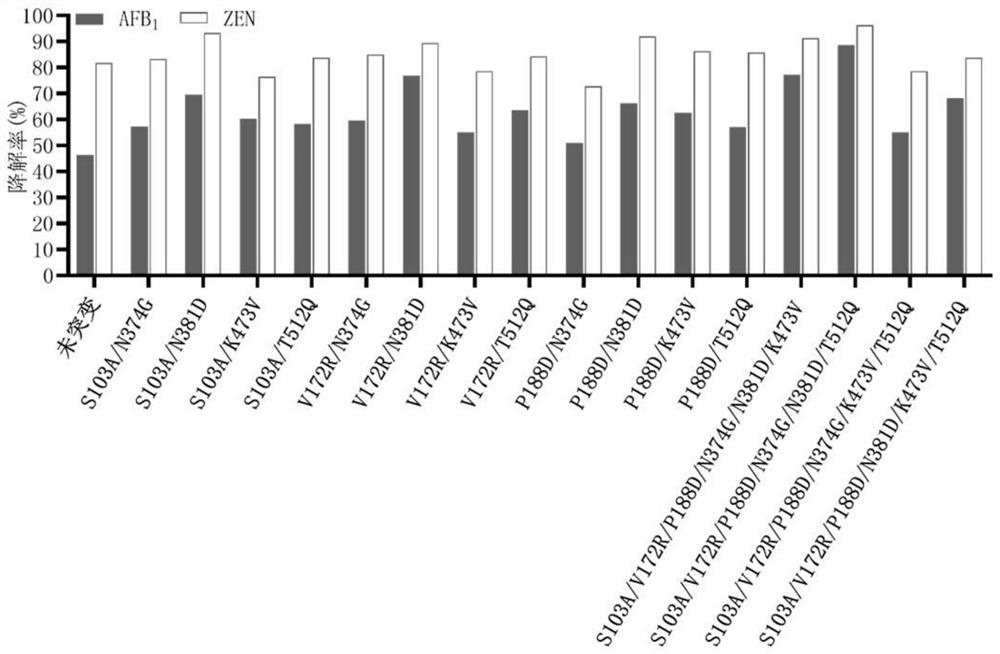
[0066] The unmutated sequence of the fusion enzyme is shown in SEQ ID NO.1. The enzyme is a fusion enzyme amino acid sequence obtained by linking the enzyme ZHD101.1 that degrades zearalenone from Clonostachys rosea and the manganese peroxidase PhcMnp enzyme from Phanerochaete chrysosporium through a unit of flexible linking peptide GGGGS As shown in SEQ ID NO.1. Mutation hotspots were determined by analysis, and the following sites were selected and correspondingly mutated: Q45V, S103A, H125R, H125E, H134D, H134A, V172R, V172T, P188D, P188H, G205Q, W210D, W210E, T211A, H248D, H248E, D250H, R301A、E328A、I334L、H339D、N374G、N374A、N374L、S379I、S379A、N381D、L407V、K443S、R447E、F448M、S461A、H466D、H466P、H466Y、V468I、R470A、K473V、K473E、V474I、T512Q、T512P、 L569M.
[0067] The specific steps are: construct the recombinant plasmid pKLAC1-zpf1: connect the nucleotide sequence encoding the fusion protei...
Embodiment 2
[0078] Example 2 Construction and Identification of Fusion Enzyme Mutant Recombinant Yeast
[0079] The recombinant plasmid constructed in Example 1 was linearized with restriction endonuclease SacII, and the digested product was purified and recovered; the linearized plasmid was transformed into Kluyveromyces lactis GG799 competent cells by electric pulse method; Immediately add 1.0 mL of pre-cooled sorbitol solution to the yeast cells after electric shock, incubate at 30°C for 1-3 hours, collect the yeast cells by centrifugation, leave 100 μL of resuspended cells, and evenly spread them on the YCB plate containing acetamide. Cultivate at ℃ for 3-5 days until a single clone grows. Pick multiple well-growing single colonies, inoculate them in 10.0 mL YEPD liquid test tubes, and culture overnight at 30°C and 200 rpm. The bacteria were collected by centrifugation, and the genome of the recombinant bacteria was extracted using a fungal genome extraction kit. The extracted genom...
Embodiment 3
[0080] The secretion expression method of embodiment 3 fusion enzyme mutant and mutant to aflatoxin B 1 and the degradation method of zearalenone
[0081] The recombinant yeast glycerol tubes carrying mutant genes that were verified to be correct by sequencing and stored in Example 2 were taken out for activation. Pick a single colony in 10.0mL YEPD medium, culture at 30°C and 200rpm for 18-22h; when OD 600When it reached 1.0, it was transferred to an inoculum containing 0.5mmol / L MnSO with 1% (1mL / 100mL) 4 and 0.2mmol / L hemin in YEPG induction medium, induce enzyme production (secretory expression) at 30°C, 200rpm for 72h. Centrifuge at 8000rpm after the end of fermentation, collect the fermentation supernatant, and concentrate it with an ultrafiltration centrifuge tube with a molecular weight cut-off of 10kDa. Protein quantification was performed on the concentrated fermentation supernatant with a protein quantification kit.
[0082] Degradation of Aflatoxin B Using Secr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com