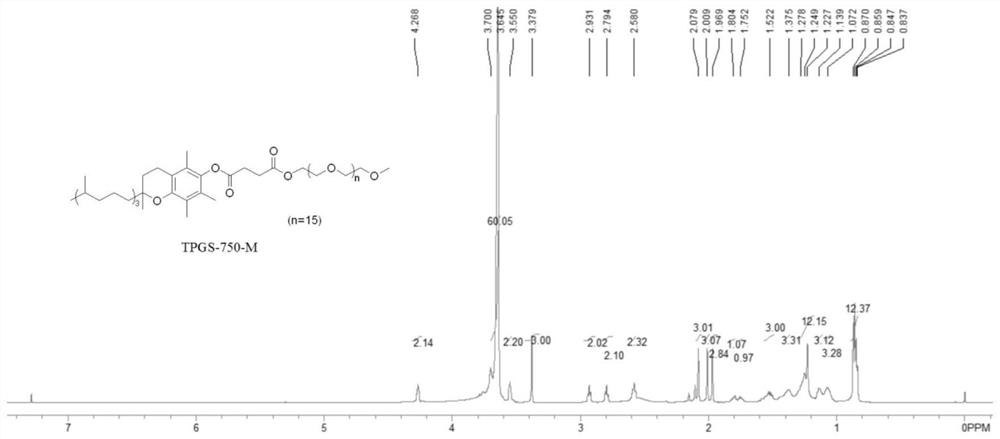
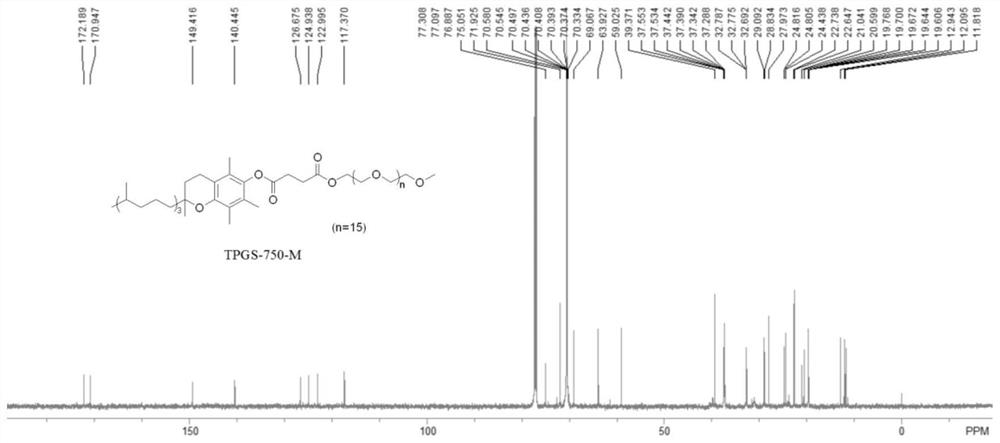
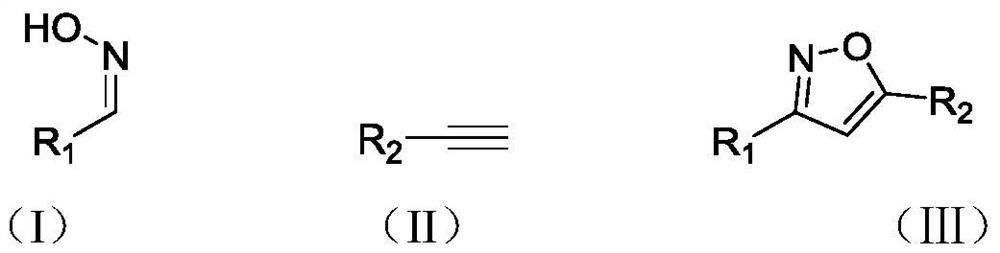Green preparation method of water-soluble vitamin E participated isoxazole compound
A technology of alkyne compounds and compounds, which is applied in the field of green synthesis of isoxazole compounds, can solve the problems of excessive waste and large pollution, and achieve the effects of efficient reaction conditions, zero emissions, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Synthesis of 3,5-diphenylisoxazole
[0043]
[0044] Add 24.1mg (0.18mmol) NCS, 18.2mg (0.15mmol) benzaldoxime (I-1) in the reaction bottle of 5mL, then add 1mL newly prepared 2wt.% TPGS-750-M aqueous solution in the bottle, The mixture was stirred at room temperature for 4 hours. Four hours later, add 14 μL (0.1 mmol) Et 3 N, 10.2mg (0.1mmol) phenylacetylene (II-1), react at room temperature for 8h. After the reaction, add 2 mL of ethyl acetate to the reaction flask, stir for 5 min, and then let stand to separate layers. After collecting the upper organic phase, add 2 mL of ethyl acetate to the lower aqueous phase, stir for 5 min, and let stand for layering. Collect the upper organic phase. Finally, the organic phases obtained twice were combined, the solvent was evaporated under reduced pressure, the solid residue was separated by column chromatography, the eluent was petroleum ether: ethyl acetate = 1:2, the eluent containing the target product was co...
Embodiment 2
[0047] Example 2: Synthesis of 3,5-diphenylisoxazole
[0048] Add 24.1mg (0.18mmol) NCS, 18.2mg (0.15mmol) benzaldoxime (I-1) in the reaction bottle of 5mL, then add 1mL newly prepared 2wt.% TPGS-750-M aqueous solution in the bottle, The mixture was stirred at room temperature for 4 hours. Four hours later, 7 μL (0.05 mmol) Et was added sequentially 3 N, 10.2mg (0.1mmol) phenylacetylene (II-1), react at room temperature for 14h. After the reaction, add 2 mL of ethyl acetate to the reaction flask, stir for 5 min, and then let stand to separate layers. After collecting the upper organic phase, add 2 mL of ethyl acetate to the lower aqueous phase, stir for 5 min, and let stand for layering. Collect the upper organic phase. Finally, the organic phases obtained twice were combined, the solvent was evaporated under reduced pressure, and the solid residue was separated by column chromatography. The eluent was petroleum ether: ethyl acetate (v / v)=1:2, and the fraction containing the...
Embodiment 3
[0049] Example 3: Synthesis of 3,5-diphenylisoxazole
[0050] Add 24.1mg (0.18mmol) NCS, 18.2mg (0.15mmol) benzaldoxime (I-1) in the reaction bottle of 5mL, then add 1mL newly prepared 2wt.% TPGS-750-M aqueous solution in the bottle, The mixture was stirred at room temperature for 4 hours. After four hours, add 21μL (0.15mmol) Et 3 N, 10.2mg (0.1mmol) phenylacetylene (II-1), reacted at room temperature for 7h. After the reaction, add 2 mL of ethyl acetate to the reaction flask, stir for 5 min, and then let stand to separate layers. After collecting the upper organic phase, add 2 mL of ethyl acetate to the lower aqueous phase, stir for 5 min, and let stand for layering. Collect the upper organic phase. Finally, the organic phases obtained twice were combined, the solvent was evaporated under reduced pressure, the solid residue was separated by column chromatography, the eluent was petroleum ether: ethyl acetate = 1:2, the eluent containing the target product was collected, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com