Method for synthesizing glabridin
A technology for synthesizing licorice and licorice, which is applied in the field of organic synthesis and fine chemicals, can solve the problems of high price, limitation of licorice harvesting, expensive boric acid reagent and metal palladium catalyst, etc., and achieve high reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
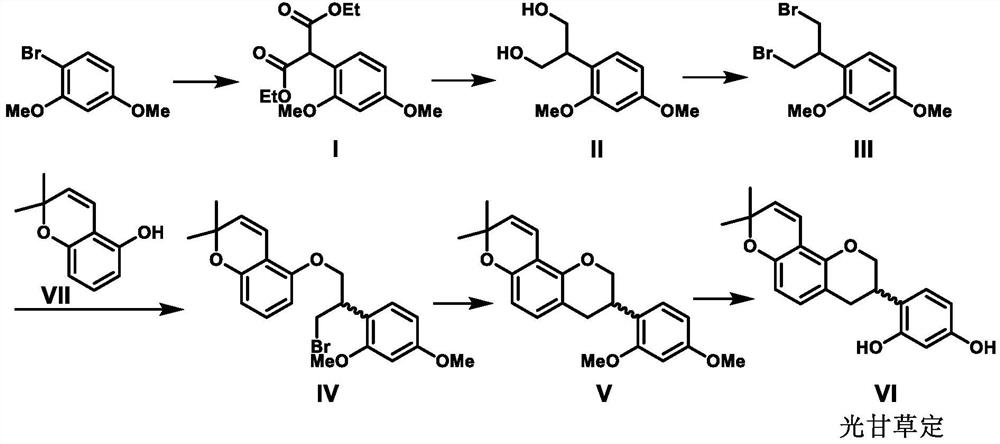
[0033] 1. Synthesis of Compound I:
[0034] Under argon atmosphere, 2,4-dimethoxybromobenzene (200 g, 0.92 mol) was dissolved in 1,4-dioxane (1 L), and cesium carbonate (330 g, 1.01 mol) was added successively , cuprous iodide (3.8 grams, 0.02 moles), 2-pyridinecarboxylic acid (4.9 grams, 0.04 moles) and diethyl malonate (162 grams, 1.01 moles), stirred at 100 ° C for 16 hours, and TLC detected the raw materials Consumed completely. The reaction solution was poured into ice water (1 liter), stirred for 0.5 hours, separated, the aqueous phase was extracted with ethyl acetate (200 ml×3), the organic phases were combined, washed with saturated brine (200 ml×1), The organic phase was concentrated until a large amount of solid precipitated, and compound I was obtained by filtration (254 g, yield 93%, white solid, melting point 53-55° C.). 1 H NMR (CDCl 3 ,400MHz): δ7.25(1H,br s,Ar-H),6.50(1H,dd,J=6.6,1.6Hz,Ar-H),6.46(1H,dd,J=1.6Hz,Ar-H ),5.02(1H,s,Ar-CH),4.27-4.17(4H,m,-CH 2 C...
Embodiment 2
[0047] The other steps are the same as in Example 1, except that in step 1, 1,4-dioxane is replaced by tetrahydrofuran; cesium carbonate is replaced by potassium carbonate; in step 4, N,N-dimethyl formazan Amide is replaced by dimethyl sulfoxide; in step 5, iron trichloride is replaced by aluminum trichloride.
Embodiment 3
[0049] Other steps are the same as in Example 1, except that in step 5, dichloromethane is replaced by nitromethane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com