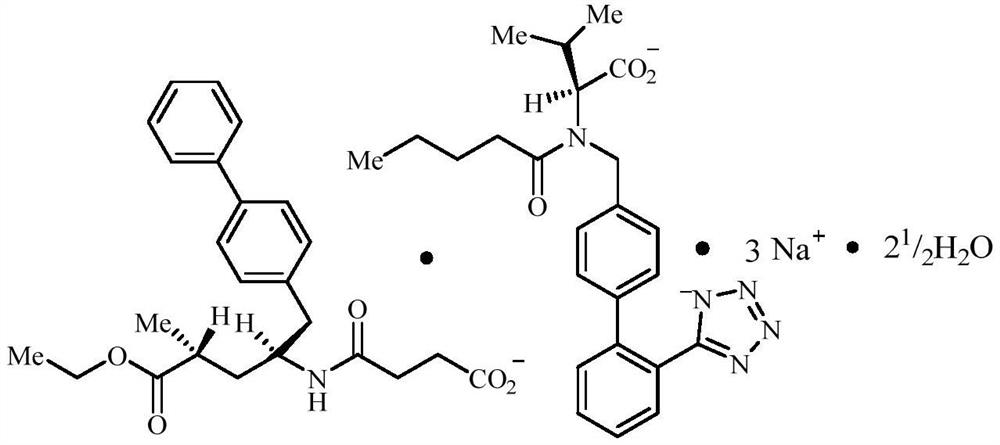
Tablet containing sacubitril valsartan sodium and preparation method thereof
A technology of sacubitrilvaler and sartan sodium, which is applied in the direction of medical preparations containing active ingredients, medical preparations without active ingredients, pill delivery, etc., which can solve the problems of easy weathering and deliquescence, stickiness without inspection, and personnel Large damage and other problems, achieve the effect of one-sided smoothness and non-sticky flushing, ensure bioavailability, and solve the problem of sticky flushing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
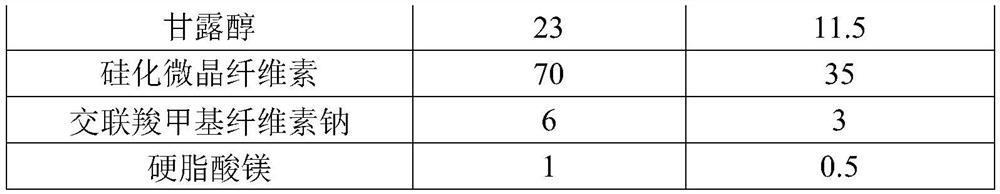
[0042] A tablet containing sacubitril-valsartan sodium, wherein the formula composition and mass percentage of the tablet core are shown in Table 1 below, wherein the mass ratio of xylitol to silicified microcrystalline cellulose is 3.0:1.
[0043] Formula composition and mass percent of table 1 tablet core
[0044] formula Dosage (g) Mass percentage (%) Sacubitril Valsartan Sodium 100 50 Xylitol 23 11.5 Silicified microcrystalline cellulose 70 35 Croscarmellose Sodium 6 3 Magnesium stearate 1 0.5
[0045] The preparation method is as follows:
[0046] (1) Sacubitril-valsartan sodium and silicified microcrystalline cellulose (D90=70 μm) were put into the hopper mixer, and mixed for 20 minutes at a speed of 15 rpm to make them uniformly dispersed;
[0047] (2) add xylitol in the mixture that obtains in step (1), with the rotating speed of 15 revs / min, mix 15 minutes, make it disperse;
[0048] (3) Add croscarmellose sodium a...
Embodiment 2
[0051] A tablet containing sacubitril-valsartan sodium, wherein the formula composition and mass percentage of the tablet core are shown in Table 2 below, wherein the mass ratio of xylitol to silicified microcrystalline cellulose is 1.8:1.
[0052] Formula composition and mass percent of table 2 tablet core
[0053] formula Dosage (g) Mass percentage (%) Sacubitril Valsartan Sodium 100 50 Xylitol 33 16.5 Silicified microcrystalline cellulose 60 30 Croscarmellose Sodium 6 3 Magnesium stearate 1 0.5
[0054] The preparation method is as follows:
[0055] (1) Put sacubitril-valsartan sodium and silicified microcrystalline cellulose (D90=120 μm) into the hopper mixer, and mix for 20 minutes at a speed of 15 rpm to make them uniformly dispersed;
[0056] (2) add xylitol in the mixture that obtains in step (1), with the rotating speed of 15 revs / min, mix 15 minutes, make it disperse;
[0057] (3) Add croscarmellose sodium and mag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com