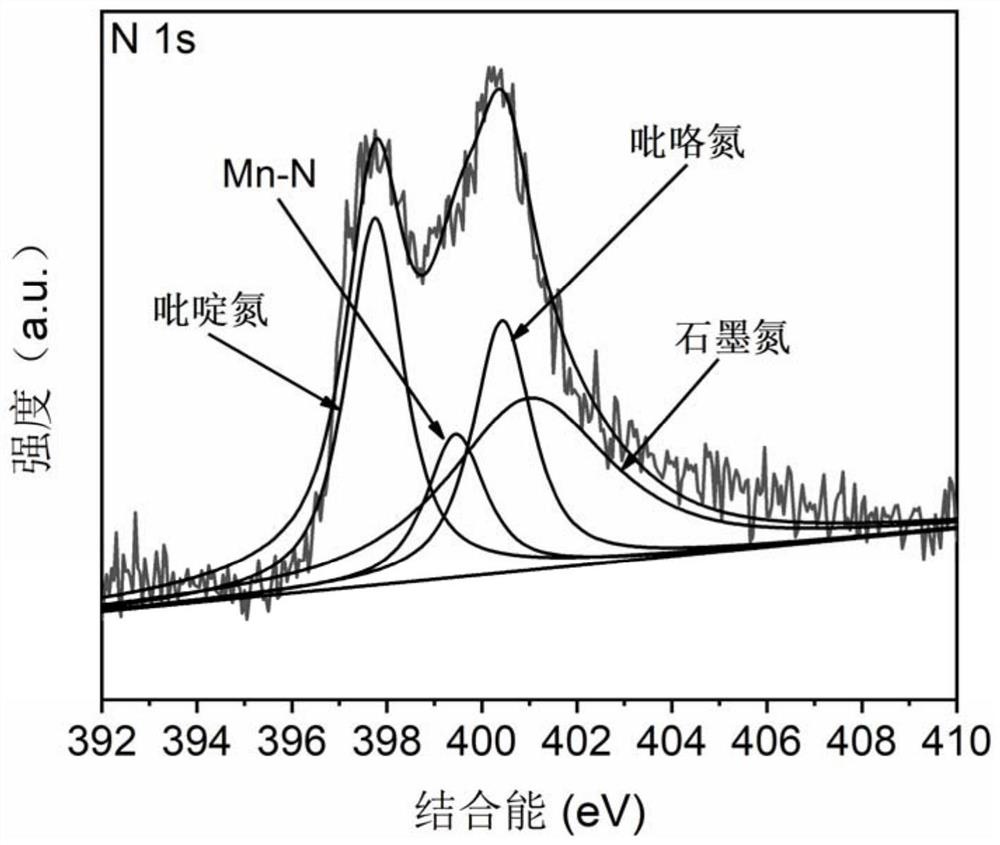
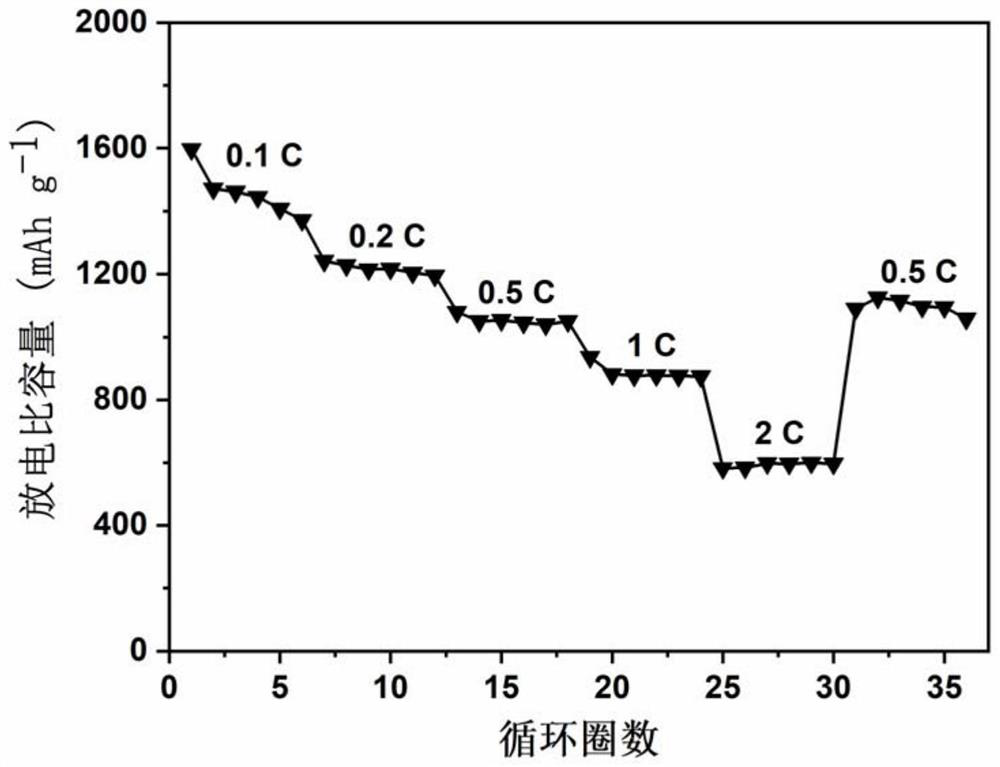
Preparation method of high-load Mn-N active site doped carbon material catalyst and application of high-load Mn-N active site doped carbon material catalyst in lithium-sulfur battery
A technology of active sites and catalysts, applied in the field of electrochemistry, can solve problems such as the difficulty of preparing catalysts, and achieve the effects of improving specific capacity and cycle stability, excellent conductivity, and improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0038] Embodiment 1 Case (prepared as schematic flow diagram Figure 5 Disted):
[0039] The first step, synthesis of Mn-ZIF-8 precursor
[0040] Take 30mL2- methylimidazole methanol concentration A 1.2mmol / ml; and zinc nitrate hexahydrate takes manganese acetate and 30mL methanol was added two concentrations of 0.2mmol / ml and 0.05mmol / ml, was stirred for 30min to give solution B. At room temperature, the solution B was added to solution A slowly stirred 50min, the resulting precipitate was allowed to stand 24h washed several times with ethanol, and dried in vacuo at 70 deg.] C 14h to give Mn-ZIF-8 precursor.
[0041] The second step, low-load synthetic Mn-N site catalysts
[0042] The pyrolysis of precursor Mn-ZIF-8, the first control heating rate in an argon atmosphere were 5 ℃ / min, heated to 750 deg.] C held IH; then ammonia gas temperature after 1.5h, continued argon atmosphere replaced after the temperature was raised to 950 deg.] C 2.5h, cooled to room temperature; th...
Embodiment example 2
[0055] The first step, synthesis of Mn-ZIF-8 precursor
[0056] Take 30mL2- methylimidazole methanol concentration A 1.0mmol / ml; and take zinc nitrate hexahydrate was added manganese acetate and 30mL methanol was both concentration 0.18mmol / ml and 0.04mmol / ml, was stirred for 30min to give solution B. At room temperature, the solution B was added to solution A, stirring slowly after 30min, allowed to stand 20h obtained was washed precipitated with ethanol several times, and dried in vacuo at 60 ℃ 12h to give Mn-ZIF-8 precursor.
[0057] The second step, low-load synthetic Mn-N site catalysts
[0058] The pyrolysis of precursor Mn-ZIF-8, the first controlled argon atmosphere were a temperature rise rate 3 ℃ / min, heated to 750 deg.] C held 0.5H; then ammonia gas temperature after 1.5h, replaced with argon gas atmosphere after the temperature was raised to 900 deg.] C thermostat continues 2.5h, cooled to room temperature; the product was obtained at 70 ℃ for using 0.5mol / LH...
Embodiment example 3
[0069] The first step, synthesis of Mn-ZIF-8 precursor
[0070] Take 30mL2- methylimidazole methanol concentration A 1.4mmol / ml; and take zinc nitrate hexahydrate was added manganese acetate and 30mL methanol was both concentration 0.22mmol / ml and 0.06mmol / ml, was stirred for 30min to give solution B. At room temperature, the solution B was added to solution A, stirring slowly after 60min, allowed to stand 26h obtained was washed precipitated with ethanol several times, and dried in vacuo at 80 ℃ 16h to give Mn-ZIF-8 precursor.
[0071] The second step, low-load synthetic Mn-N site catalysts
[0072] The pyrolysis of precursor Mn-ZIF-8, the first control heating rate in an argon atmosphere were 5 ℃ / min, holding temperature was raised to 750 deg.] C for 1.5 h; then ammonia gas temperature after 2h, continued argon atmosphere replaced after the temperature was raised to 950 deg.] C 2.5h, cooled to room temperature; the product was obtained at 80 ℃ for using 0.5mol / LH 2 SO ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com