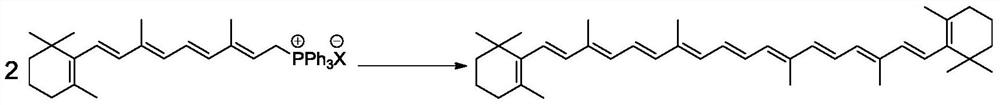
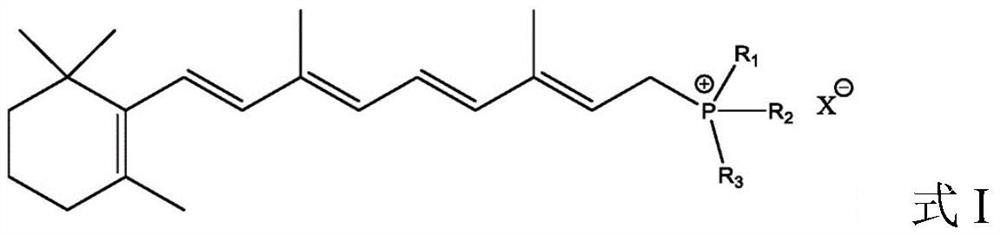
Preparation method of beta-carotene
A carotene and vitamin technology, applied in the direction of organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problems that β-carotene cannot be produced in time, β-carotene is easily oxidized, and is not suitable for industrial production. To achieve the effects of inhibiting oxidation side reactions, low requirements, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] [Preparation Example 1] Preparation of KBO 3 4H 2 O solid
[0044] Weigh 8.1g of KBO 2 Solid, dissolved in 100mL of distilled water, adding 5g of magnesium silicate to the solution and stirring at a constant temperature of 15°C for 30min; keeping the temperature for 30min, adding 11.34g of 30% hydrogen peroxide aqueous solution to the reaction system; Lower the temperature to below 0°C, filter and dry to obtain KBO 3 4H 2 O solid.
Embodiment 2
[0045] [Preparation Example 2] Preparation of LiBO 3 4H 2 O solid
[0046] Weigh 5g of LiBO 2 Solid, dissolved in 100mL of distilled water, add 6g of magnesium silicate to the solution and stir at a constant temperature of 15°C for 30min; keep the temperature for 30min and add 11.34g of 30% hydrogen peroxide aqueous solution to the reaction system; after the dropwise addition, keep warm for 5h, Lower the temperature to below 0°C, filter and dry to obtain LiBO 3 4H 2 O solid.
[0047] 【Example 1】
[0048] Add 134.0g vitamin A acetate (0.4mol, 98%), 125.8g triphenylphosphine (0.48mol), 670.0g methanol to a 2L reactor, cool to 0°C while stirring, and maintain 0-5°C 44 g of concentrated sulfuric acid (0.44 mol, 98%) was slowly added, and the addition was completed dropwise in 1 h; then reacted at 25° C. for 8 h to obtain an organic phosphonium salt reaction solution.
[0049] After desolvating the obtained organic phosphine salt reaction solution, add 1206.0g of water, cool...
Embodiment 3
[0054] The vitamin A acetate in the implementation example 1 was replaced by 119.4g vitamin A alcohol, and other conditions were the same, and the organic phosphonium salt reaction solution was obtained.
[0055] After desolvating the obtained organic phosphine salt reaction solution, add 1206.0g of water, cool down to 5°C, and add 126.9g of NaBO to the reaction system 3 4H 2 O (0.8mol, 97%) was reacted for 12h; the reaction solution was filtered, 300g of methanol was added to the obtained filter cake and refluxed for 2h, and then filtered and dried to obtain 99.5g of β-carotene. The purity by HPLC was 98.0%, and the total yield was 91.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com