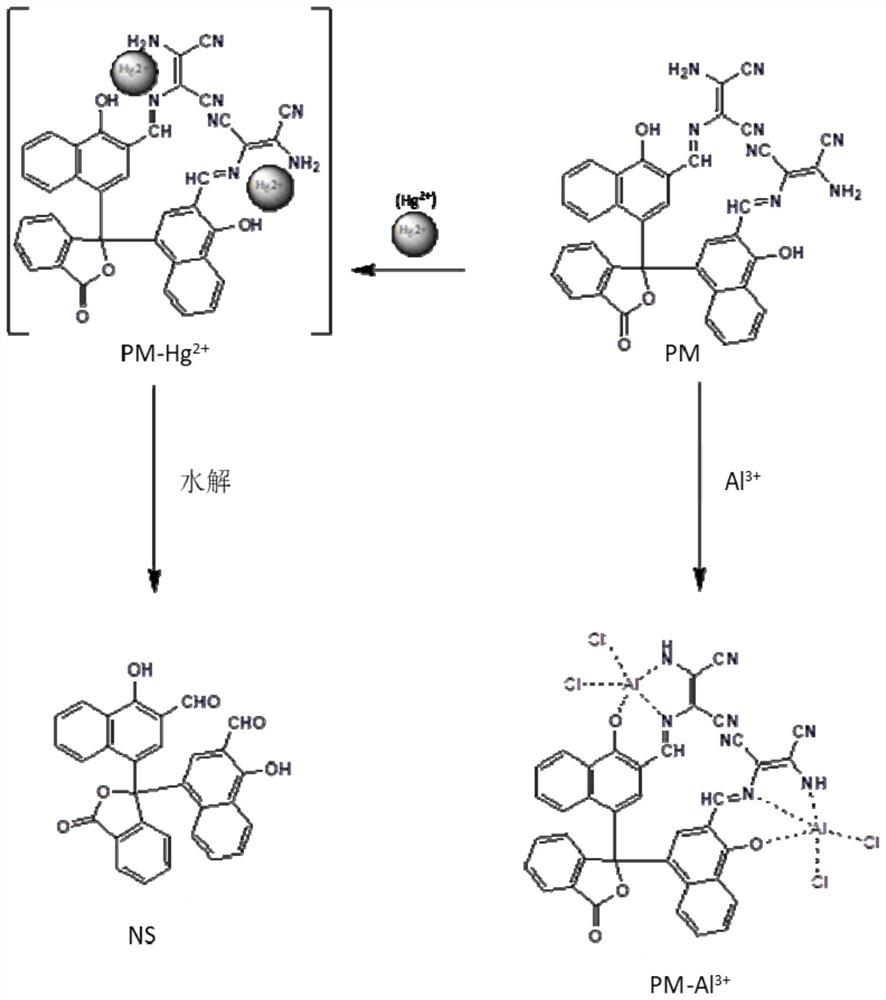
Alpha-naphtholphthalein derivative multifunctional fluorescent probe as well as preparation method and application thereof
A technology of fluorescent probes and derivatives, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of complex synthesis steps, low sensitivity, large molecular weight, etc., and achieve strong anti-interference ability and simple preparation method High efficiency and excellent selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The steps of the preparation method of the above-mentioned α-naphtholphthalein derivatives multifunctional fluorescent probe are as follows:
[0047] (1) The compound of formula (III) is dissolved in trifluoroacetic acid, then hexamethylenetetramine is added, and the compound of formula (II) is generated under nitrogen atmosphere at 70-110°C, with a yield of 50%-80%.
[0048](2) Dissolving the compound of formula (II) in a mixed solvent of dichloromethane and ethanol, then adding diaminomaleonitrile (diaminomaleonitrile) dissolved in a mixed solvent of dichloromethane and ethanol, while dropping After adding glacial acetic acid, and reacting for 2-8 hours, after extraction and column chromatography separation and purification, α-naphtholphthalein derivatives multifunctional fluorescent probes are obtained with a yield of 65%-85%.
[0049] The present invention also provides a method for detecting Hg using α-naphtholphthalein derivatives multifunctional fluorescent probe...
Embodiment 1
[0052] Example 1 Synthesis of α-naphtholphthalein derivatives multifunctional fluorescent probe (I)
[0053] The reaction formula of synthetic α-naphtholphthalein derivatives multifunctional fluorescent probe (I) is as follows:
[0054]
[0055] The specific steps of synthetic α-naphtholphthalein derivatives multifunctional fluorescent probe (I) are as follows:
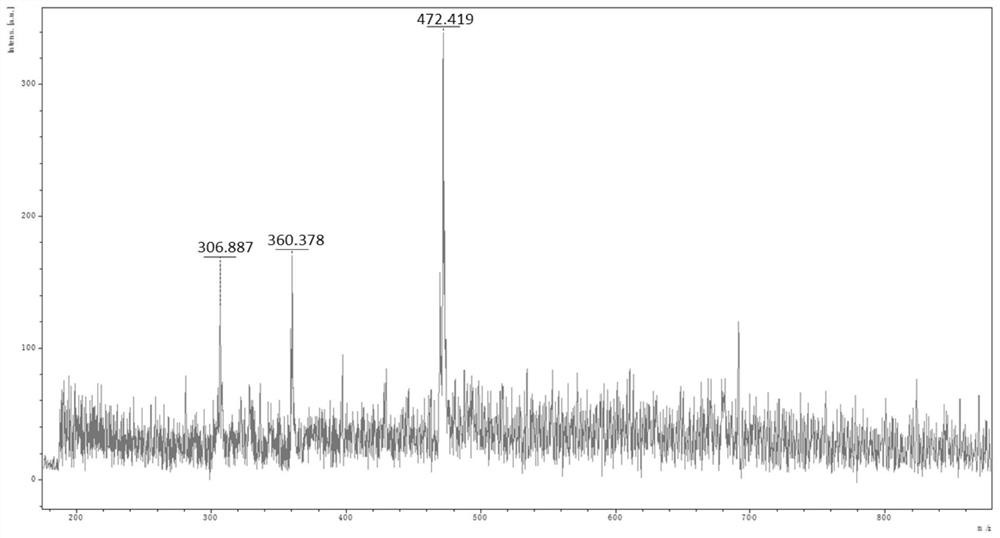
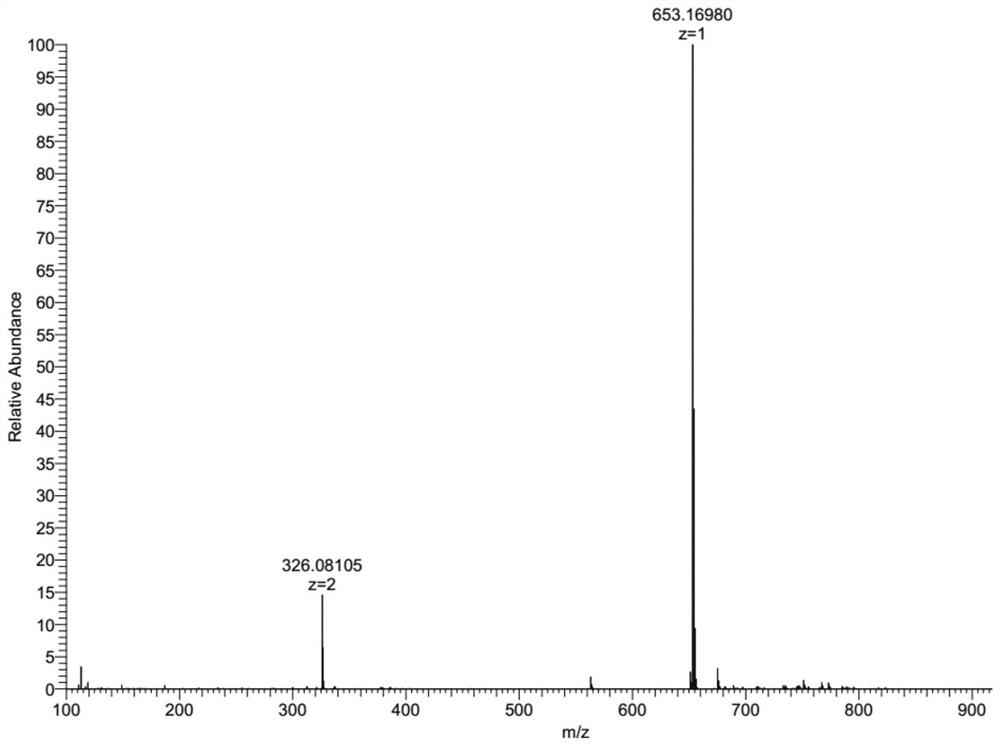
[0056] (1) Dissolving the formula (III) in trifluoroacetic acid, and then gradually adding urotropine (hexamethylenetetramine). Under the protection of nitrogen, heat and reflux at 70-110° C. for 2-8 hours, and then cool the reaction system to room temperature. Separate, wash, dry, and filter after standing. The resulting filtrate was spin-dried with a rotary evaporator to obtain a crude product; finally, the crude product was separated and purified by column chromatography and then spin-dried with a rotary evaporator to obtain the pure product formula (II), with a yield of about 50% to 80%. The mass spectrometr...
Embodiment 2
[0058] Embodiment 2 fluorescent probe of the present invention is paired with Hg 2+ and Al 3+ selective detection of
[0059] 10 μmol / L α-naphtholphthalein derivatives multifunctional fluorescent probe (fluorescent probe (I) prepared in embodiment 1) dimethyl sulfoxide and water have a volume ratio of 9:1 mixed solution, add 100 μmol / L respectively Metal ions of L (Zn 2+ ,Mg 2+ , Ca 2+ ,Co 2+ ,Fe 3+ , Ni 2+ ,Sn 2+ ,Cd 2+ , Li + , Na + ,Pb 2+ ,Mn 2+ , Fe 2+ ,VO 2+ ,Cu 2+ ). After 15 minutes, detect the changes in the fluorescence emission spectrum and the ultraviolet-visible absorption spectrum of the solution, and the detection results are as follows: Figure 4 and Figure 5 shown. The α-naphtholphthalein derivatives multifunctional fluorescent probe has almost no emission peaks at 520nm and 480nm, and almost no absorption peak at 436nm. When Al 3+ After that, the α-naphtholphthalein derivatives multifunctional fluorescent probe showed a strong emission pea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com