Synthesis method of dydrogesterone
A synthetic method, pregnant technology, applied in the field of drug synthesis, can solve the problems of many steps, low yield, lengthy synthetic route steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
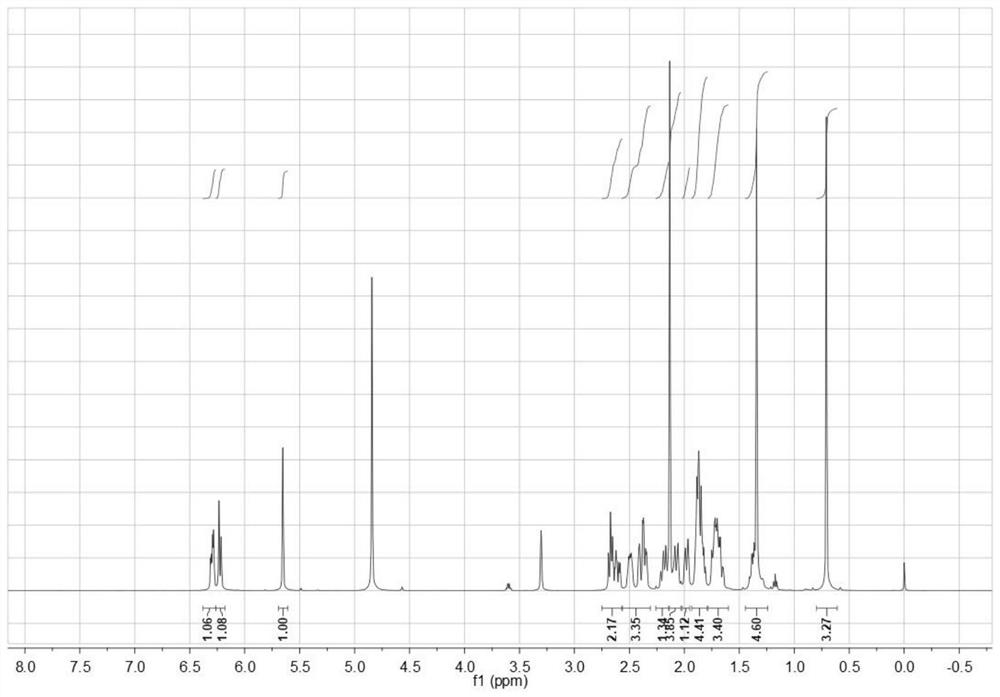
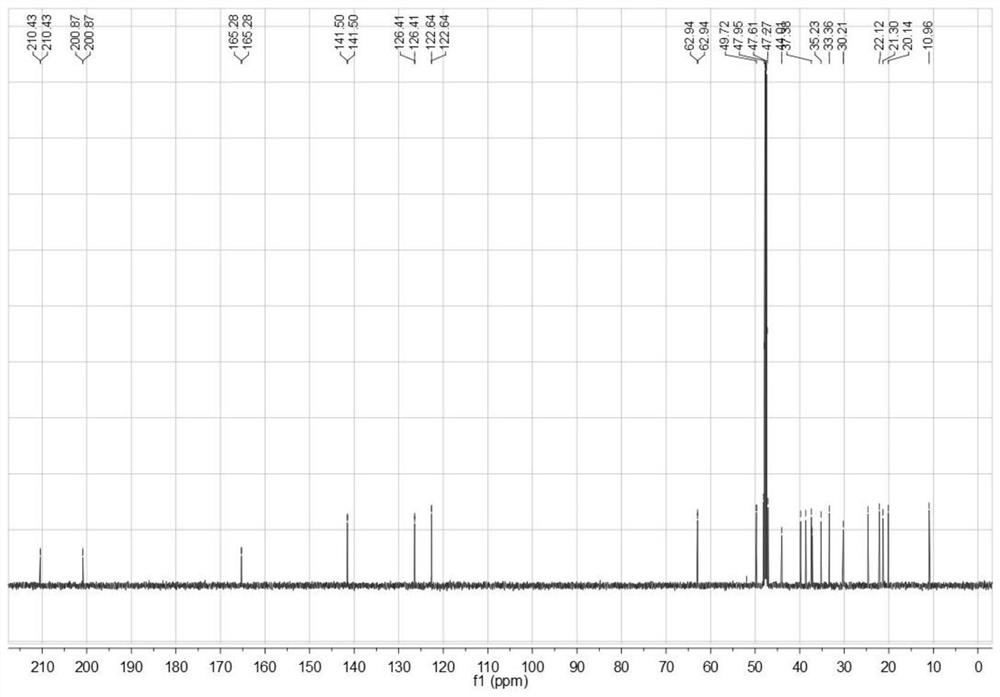
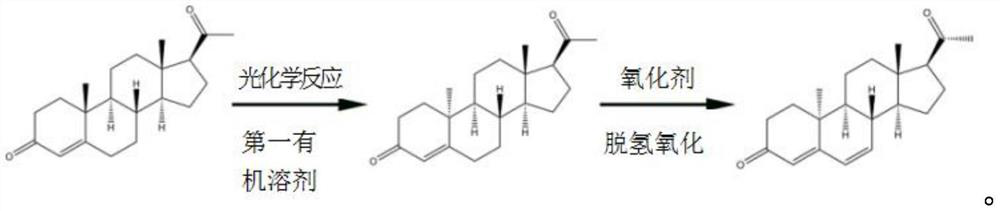
[0024] The invention provides a kind of synthetic method of dydrogesterone, comprising the following steps:
[0025] Dissolve progesterone and perform photochemical reaction to obtain 9β,10α-pregna-4-ene-3,20-dione;
[0026] mixing the 9β,10α-pregn-4-ene-3,20-dione, an organic solvent and a benzoquinone oxidizing agent, and performing a dehydrogenation reaction to obtain dydrogesterone;
[0027] The organic solvents include alcohol organic solvents, ether organic solvents and aromatic hydrocarbon organic solvents.
[0028] In the present invention, unless otherwise specified, the raw materials used in the present invention are preferably commercially available products.
[0029] The invention dissolves the progesterone and performs photochemical reaction to obtain 9β, 10α-pregna-4-ene-3,20-dione.
[0030] In the present invention, the dissolved reagent preferably includes a polar organic solvent, and the polar organic solvent preferably includes one or more of tetrahydrofura...
Embodiment 1
[0059] Under the protection of nitrogen, mix 1.6g of progesterone and 250mL of tetrahydrofuran to carry out the first photochemical reaction and the second photochemical reaction. The condition of the first photochemical reaction is to use a high-pressure mercury lamp for 1h, and the wavelength is 200-400nm; The condition of the photochemical reaction is to place the pyrex glass tube around the high-pressure mercury lamp, filter out the light with a wavelength less than 300nm, and continue to irradiate for 3h.
[0060] Evaporate the solvent in the system after the photochemical reaction to dryness, add 150 mL of ethanol / acetonitrile = 3:1 mixed solvent to the obtained solid to redissolve, heat and reflux for 10 min at a temperature of 75 ° C, and obtain the solution at a temperature of -5 ° C Cool down for 1 hour, and after the solid precipitates, perform suction filtration, and vacuum-dry the product obtained by suction filtration at a vacuum degree of -0.07MPa and a temperatu...
Embodiment 2
[0071] Example 2 According to the synthesis method of Example 1, the only difference is that the light source is replaced by a xenon lamp, and finally 0.98g of dydrogesterone is obtained, with a total yield of 82.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com