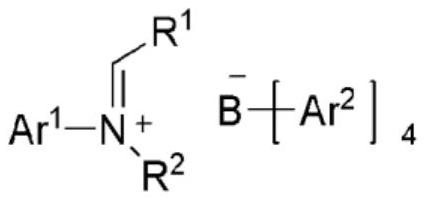
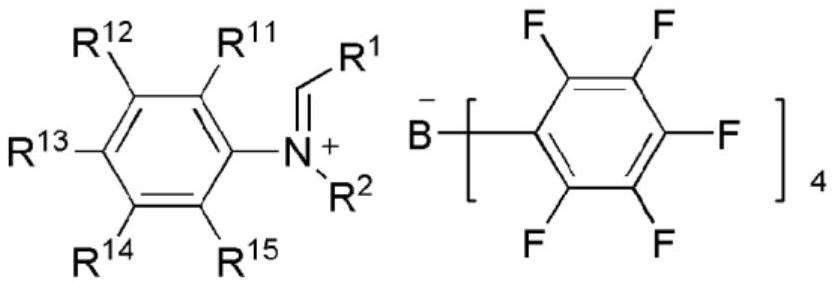
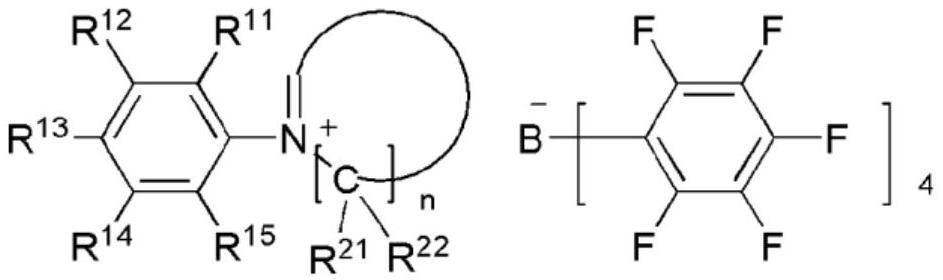
NOVEL TETRAARYLBORATE COMPOUND, CATALYST COMPOSITION COMPRISING SAME, AND METHOD FOR PREPARING ETHYLENE HOMOPOLYMERS OR COPOLYMERS OF ETHYLENE AND alpha-OLEFIN BY USING SAME
A technology of tetraaryl borate and aryl borate, which is applied in the field of tetraaryl borate compounds, can solve problems such as uneven composition distribution, failure, and operation interruption, and achieve reduced consumption, high yield, and promotion of The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0186] Preferred organic solvents can be used in the preparation method of the present invention may be an aliphatic hydrocarbon-based solvent, (C3-C20) hydrocarbon, and the specific example thereof may be selected from one or both or more of the following Mixed Solvent: Based on linear aliphatic hydrocarbon solvent, such as butane, isobutane, pentane, hexane, heptane, octane, isooctane, decane, decane and dodecane; and cyclic Solvents of aliphatic hydrocarbons such as cyclopentane, methylcyclopentane, cyclohexane, and methylcyclohexane.
[0187] The organic solvents that can be used in the preparation method can be an aromatic hydrocarbon-based solvent.
[0188] Specifically, when the ethylene homopolymer is prepared, ethylene is used as a monomer, wherein the suitable ethylene pressure may be 1ATM to 1000 calm, more preferably 6 atm to 150 atm. Further, the polymerization temperature of 25 ° C to 220 ° C, preferably from 70 ° C to 220 ° C, and more preferably from 100 ° C to 220...
preparation example 1
[0205] (Preparation Example 1) Preparation of complex 1
[0206] Preparation of Compound 1-A
[0207]
[0208] (Preparation Example 1) Preparation of complex 1
[0209] Preparation of Compound 1-B
[0210]
[0211] The compound 1-A (6.00 g, 31.4 mmol) was added to a 250 mL round bottom flask under a nitrogen atmosphere, and 150 ml of anhydrous tetrahydrofuran (THF) was added thereto, and the mixture was stirred. N-tert-butyl-1-chloro-1-methyl-1-phenylsilane (7.16 g, 31.4 mmol) was added by dissolving in tetrahydrofuran (THF) (50 mL), and then the mixture was stirred at room temperature 12 Hour. The solvent was removed in vacuo and dissolved by adding n-hexane (150 mL), and then the solid was removed with a filter filled with dried celite. The solvent was removed to obtain a compound 1-b (10.8 g, yield of 91.0%, and the ratio of diastereomer) was obtained.
[0212] 1 H-NMR (500MHz, C 6 Di 6 PPM): Δ0.156 (D, 3H), 0.703-0.830 (M, 1H), 0.976 (D, 9H), 1.501-1.528 (m, 4H), 3.089-3...
Embodiment 1
[0218] N- octadecyl -N- alkylene octadecylphenyl tetrakis (pentafluorophenyl) borate [C 17 Hide 35 -C = N + (C 18 Hide 37 ) (C 6 Hide 5 ) B (C 6 Fly 5 ) 4 - ] Preparation of
[0219] Was added 3g (3.25mmol) trityl tetrakis (pentafluorophenyl) borate in a glove box 1L round bottom flask. Then, 300g of cyclohexane was added to the round bottom flask, and the mixture was stirred to prepare a yellow suspension at room temperature. To the suspension was added a solution of 1.95g (3.26mmol) as solid N, N- bis octadecylphenyl amine, and then stirred at room temperature for 30 minutes. Once the suspension solution became clear, which was used in subsequent copolymerization reactions without further separation.
[0220] 1 H-NMR (500MHz, CDCl 3 , Ppm): δ0.86-0.89 (m, 6H), 1.22-1.32 (m, 62H), 1.44-1.51 (m, 2H), 3.43-3.47 (m, 2H), 7.05-7.58 (m, 5H) , 7.64-7.68 (m, 1H).
[0221] Ethylene and 1-octene copolymerization by a continuous solution process
[0222] Using a continuous polymerization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com