Cephalosporin compound and application thereof to preparation of medicine for treating diabetes and complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
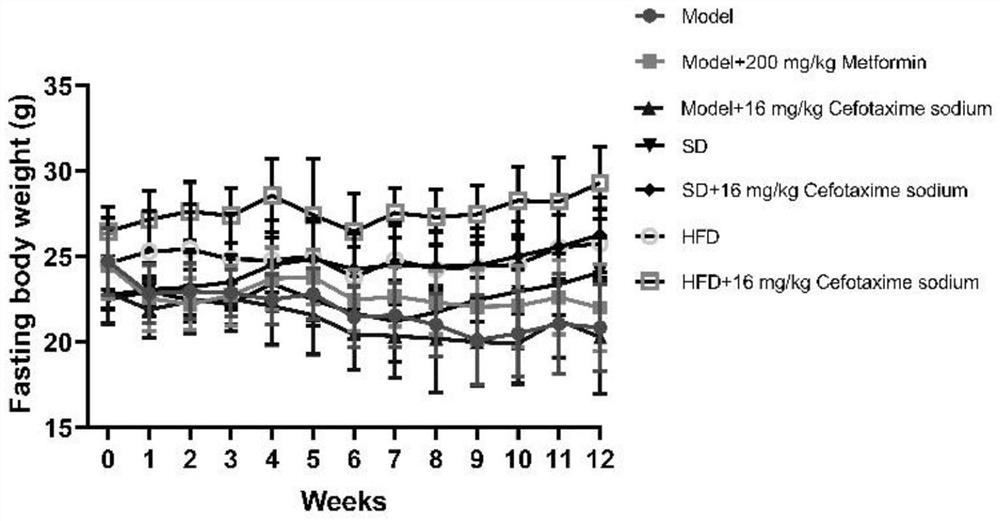
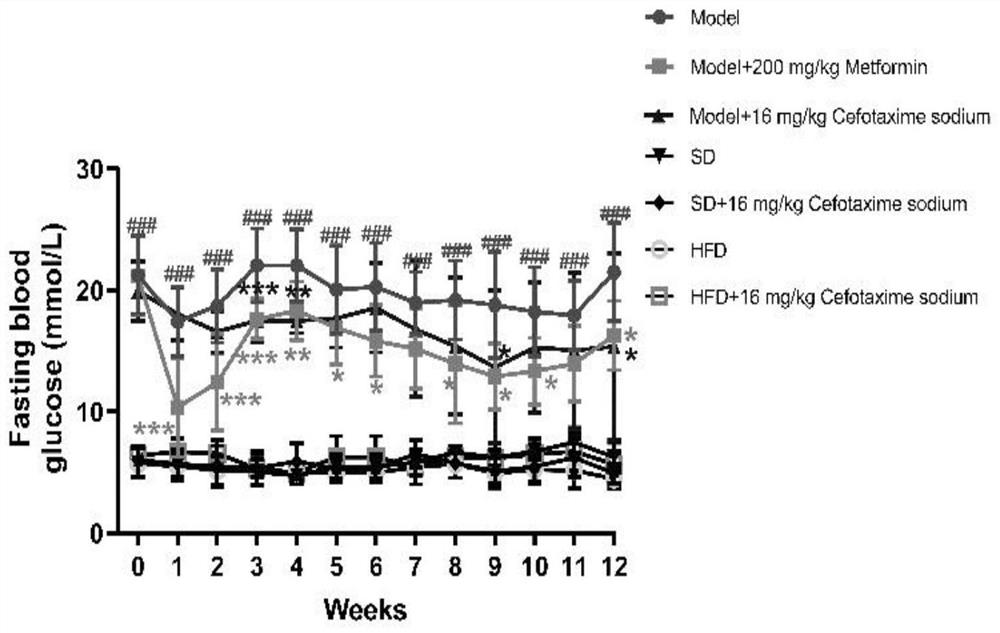
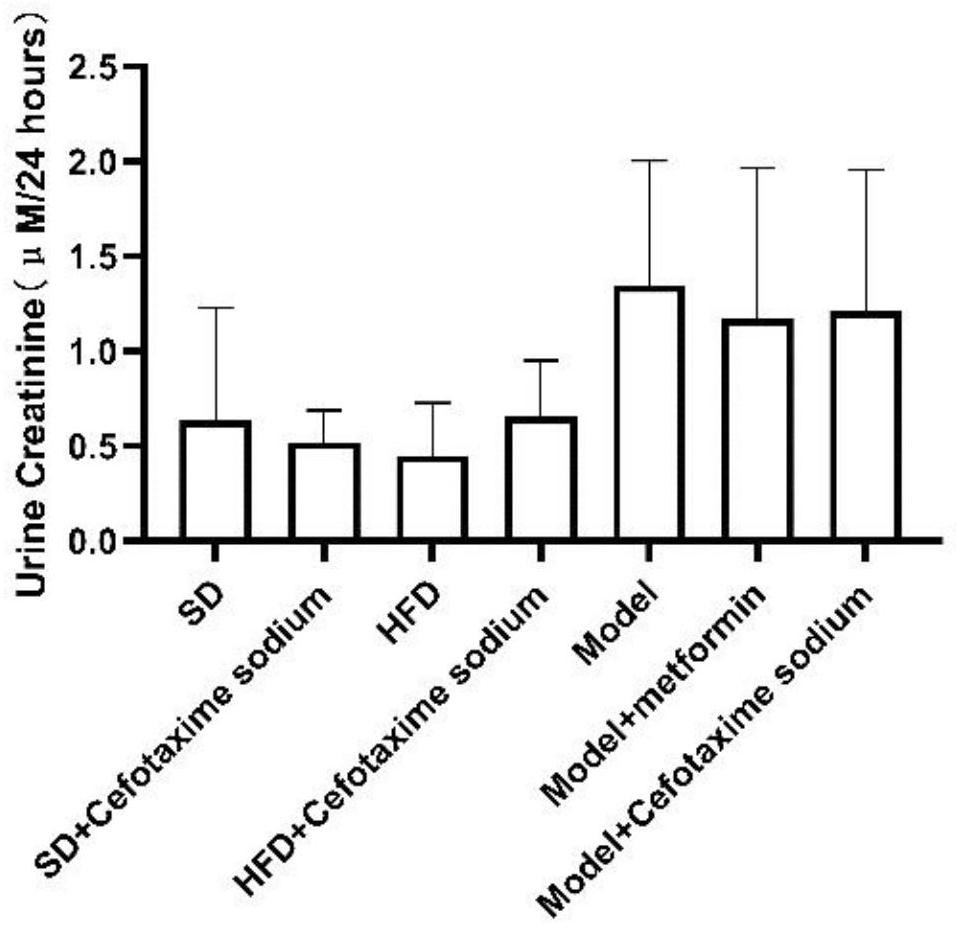
[0094] Embodiment 1, the effect of cefotaxime sodium in treating diabetes in mice and its complications
[0095] 1. Animal feeding
[0096] Experimental animals: 8-week-old SPF grade C57BL / 6N male mice, weighing 18-22 g, never used any drugs before the experiment, from Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. The experimental animals were adaptively fed for one week in an environment with a temperature of 24-26°C and a 12h / 12h alternation of day and night. The animals were given food and water freely, and then grouped experiments were carried out.
[0097]2. Drugs and reagents:
[0098] The drugs used in the present invention include: Cefotaxime sodium; Metformin hydrochloride (Metformin); Streptozocin (STZ).
[0099] Preparation of reagents: (1) Citric acid buffer solution: add 2.1g citric acid to 100mL distilled water to prepare solution A, add 2.94g sodium citrate to 100mL distilled water to prepare solution B. Mix liquid A and liq...
Embodiment 2
[0146] Embodiment two, the effect of ceftriaxone sodium in treating diabetes in mice and its complications
[0147] 1. Animal feeding method is as shown in embodiment one:
[0148] 2. Drugs and reagents:
[0149] The drugs used in the present invention include: Ceftriaxone Sodium; Metformin hydrochloride (Metformin); Streptozocin (STZ).
[0150] Preparation of reagents: (1) Citric acid buffer solution: add 2.1g citric acid to 100mL distilled water to prepare solution A, add 2.94g sodium citrate to 100mL distilled water to prepare solution B. Mix liquid A and liquid B at a ratio of 1:1.32 or 1:1, adjust the pH to 4.2-4.5, and filter the mixed solution with a 2.22 μm filter.
[0151] ⑴ Ceftriaxone sodium solution (16mg / kg): prepared with physiological saline
[0152] ⑵Metformin hydrochloride solution (200mg / kg): prepared with normal saline
[0153] 3. Establishment of type 2 diabetes model in C57BL / 6N mice
[0154] Type 2 diabetes model group (Model): 56, after three weeks ...
Embodiment 3
[0167] Embodiment three, the effect of cefodizime sodium treatment mouse diabetes and its complications
[0168] 1. Animal feeding method is as shown in embodiment one:
[0169] 2. Drugs and reagents:
[0170] The drugs used in the present invention include: Cefodizime sodium; Metformin hydrochloride (Metformin); Streptozocin (STZ).
[0171] Preparation of reagents: (1) Citric acid buffer solution: add 2.1g citric acid to 100mL distilled water to prepare solution A, add 2.94g sodium citrate to 100mL distilled water to prepare solution B. Mix liquid A and liquid B at a ratio of 1:1.32 or 1:1, adjust the pH to 4.2-4.5, and filter the mixed solution with a 2.22 μm filter.
[0172] ⑴ Cefodizime sodium solution (16mg / kg): prepared with physiological saline
[0173] ⑵Metformin hydrochloride solution (200mg / kg): prepared with normal saline
[0174] 3. Establishment of type 2 diabetes model in C57BL / 6N mice
[0175] Type 2 diabetes model group (Model): 56, after three weeks of fe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com