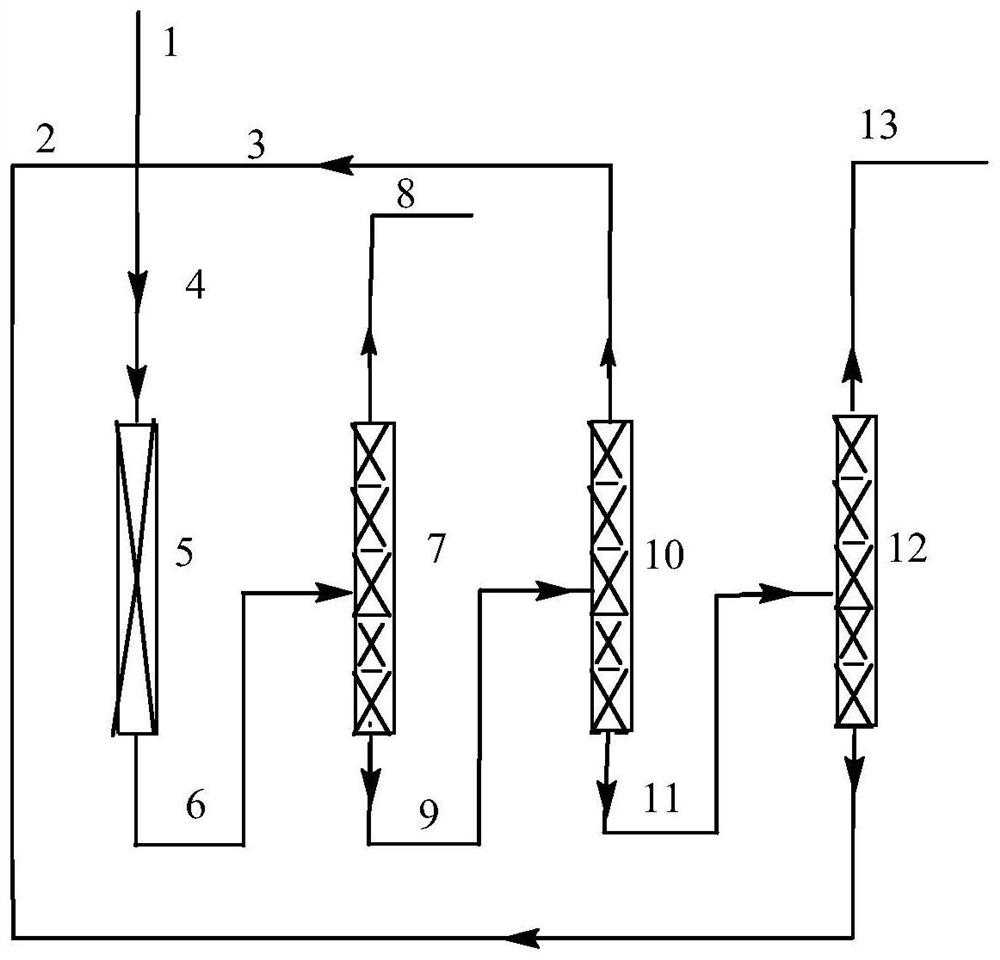
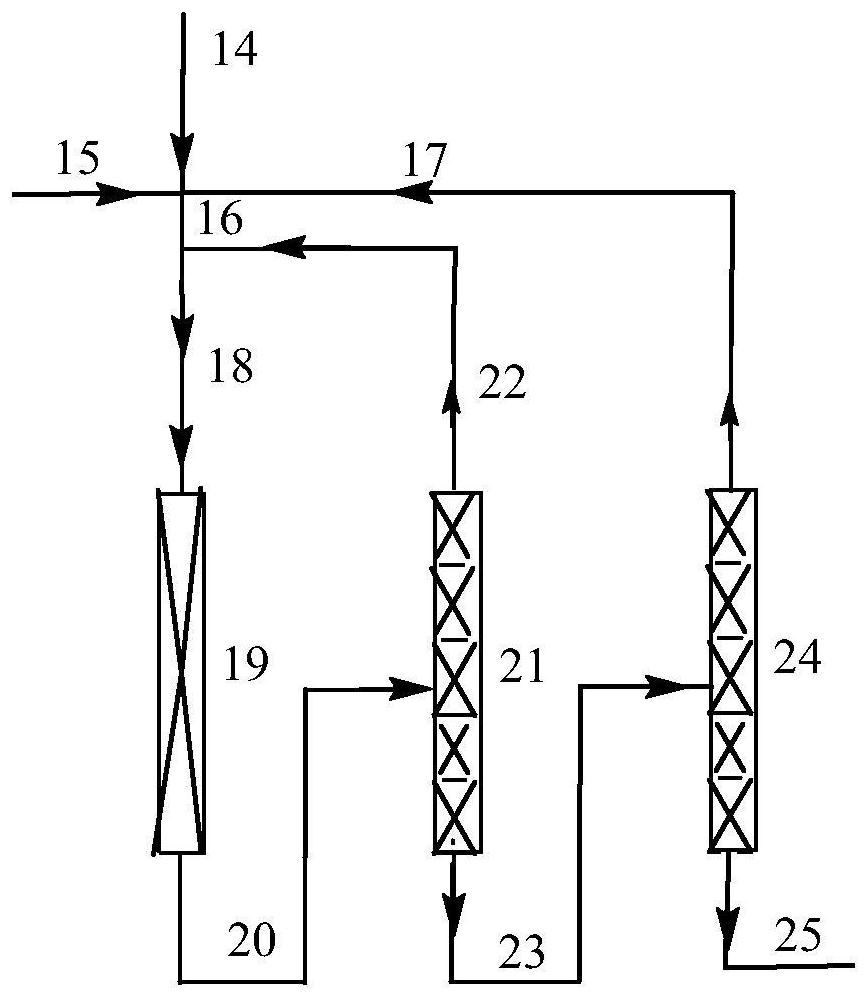
Method for preparing heptafluoroisobutyronitrile through gas-phase fluorocyaniding
A technology of heptafluoroisobutyronitrile and gas phase, which is applied in chemical instruments and methods, cyanogen halide reaction preparation, organic chemistry, etc., can solve the problems of polluting the environment, difficulty in recycling and low yield of heptafluoroisobutyronitrile, etc. To achieve the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of fluorination catalyst: according to the mass percent content of chromium ion (trivalent or / and tetravalent or / and pentavalent chromium ion) and cobalt element is 90% and 10%, chromium chloride and cobalt chloride are dissolved In water, add precipitant ammonia water at 60°C, control the pH of the solution between 7 and 9, make it fully precipitate under stirring conditions, then age for 10-24 hours, filter the formed slurry, and wash with deionized water until Neutral, then dry at 150°C for 10-24 hours to obtain a solid, crush the above-mentioned solid, press and shape to obtain a catalyst precursor, and then roast the catalyst precursor at 450°C for 10-24 hours in a nitrogen atmosphere, Activate with a mixed gas of hydrogen fluoride and nitrogen with a molar ratio of 1:2 for 10-24 hours, and oxidize for 10-24 hours at 300°C with a mixed gas atmosphere of nitrogen dioxide and nitrogen with a molar ratio of 1:10. Partially or completely converting trivalen...
Embodiment 2
[0063] The same operation as in Example 1, the difference is that "according to the mass percentages of chromium ions (trivalent or / and tetravalent or / and pentavalent chromium ions) and cobalt elements are 90% and 10%, the chlorine Dissolve chromium chloride and cobalt chloride in water" to "dissolve chromium chloride and magnesium chloride in water according to the mass percentages of chromium ions and magnesium elements being 90% and 10%. The reaction results are as follows: the conversion rate of hexafluoropropylene is 97.3%, and the selectivity of heptafluoroisobutyronitrile is 95.6%.
Embodiment 3
[0065] The same operation as in Example 1, the difference is that "according to the mass percentages of chromium ions (trivalent or / and tetravalent or / and pentavalent chromium ions) and cobalt elements are 90% and 10%, the chlorine Chromium chloride and cobalt chloride are dissolved in water " to " be 90% and 10% according to the mass percent content of chromium ion (trivalent or / and tetravalent or / and pentavalent chromium ion) and iron element, chlorine Chromium chloride and ferric chloride dissolved in water". The reaction results are as follows: the conversion rate of hexafluoropropylene is 98.7%, and the selectivity of heptafluoroisobutyronitrile is 97.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com