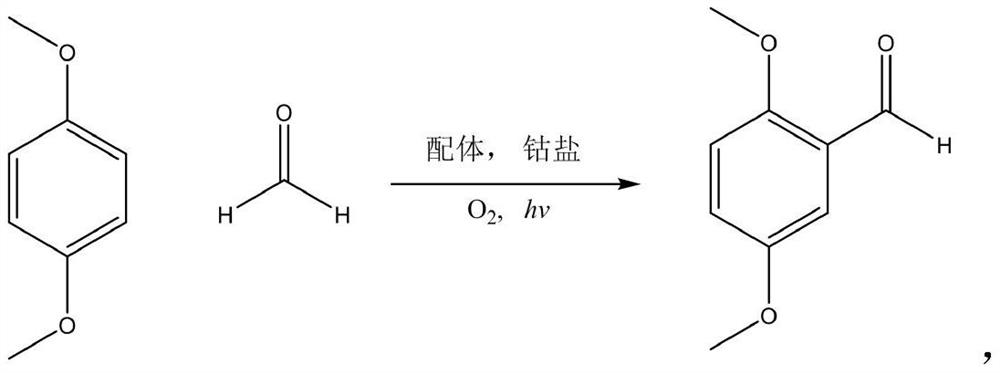
Green synthesis method of 2, 5-dimethoxybenzaldehyde
A technology of dimethoxybenzaldehyde and dimethoxybenzene is applied in chemical instruments and methods, condensation preparation of carbonyl compounds, organic compounds/hydrides/coordination complex catalysts, etc. Friendly, high operating process requirements and other issues, to achieve the effect of simple synthesis and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
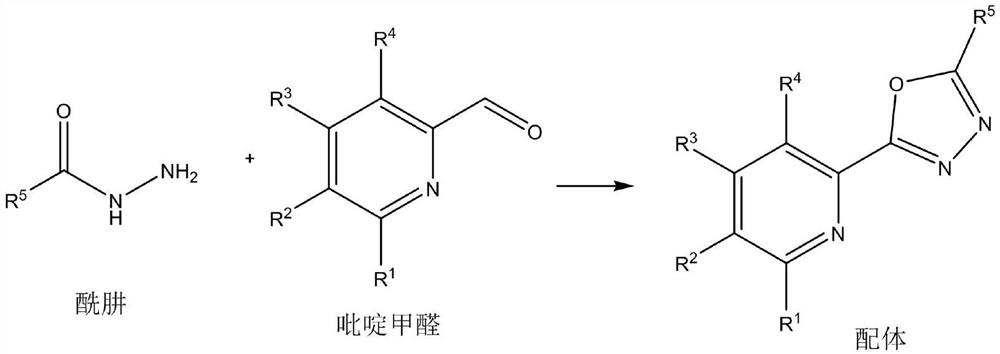
[0018] General step one (preparation of ligand):
[0019]
[0020] Add hydrazide (10mmol), pyridine formaldehyde (10mmol) and solvent methanol (20mL) into the reaction flask, add elemental iodine (0.5mmol) under stirring, and reflux reaction in the presence of air for 8 hours. After the reaction, add water and ethyl acetate The ester was extracted three times. The water layer was removed, and the organic layer was dried over anhydrous sodium sulfate, filtered, concentrated, and recrystallized with 20 mL of ethanol to obtain the ligand.
Embodiment 1
[0021] Embodiment 1 (preparation of ligand L1)
[0022]
[0023] The hydrazide is selected from acetylhydrazide, the pyridine formaldehyde is selected from pyridine-2-carbaldehyde, and the ligand L1 is prepared according to the method of general step 1. 1 H NMR: δ8.59(d, J=7.5Hz, 1H), 8.01(d, J=7.5Hz, 1H), 7.85(dd, J=7.5, 7.5Hz, 1H), 7.42(dd, J=7.5 ,7.5Hz,1H), 2.63(s,3H). 13 C NMR: δ164.7, 164.5, 157.4, 149.2, 137.2, 124.2, 123.6, 20.5.
Embodiment 2
[0024] Embodiment 2 (preparation of ligand L2)
[0025]
[0026] The hydrazide is selected from benzohydrazide, the pyridine formaldehyde is selected from 4-methoxypyridine-2-carbaldehyde, and the ligand L2 is prepared according to the method of general step 1. 1 H NMR: δ8.61(d, J=7.5Hz, 1H), 7.98(m, 2H), 7.73(d, J=7.5Hz, 1H), 7.62(m, 3H), 7.43(s, 1H), 3.81 (s,3H). 13 C NMR: δ164.8, 164.4, 159.4, 158.3, 150.2, 133.7, 129.3, 129.1, 128.7, 127.7, 127.5, 107.9, 105.4, 55.8.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com