Progesterone sustained-release composition and application thereof
A slow-release composition and a technology for progesterone, which are applied in the field of biomedicine, can solve the problems of affecting the stability of drug release of long-acting preparations, unable to adapt to clinical needs of progesterone, unfavorable product quality control, etc. The gel structure is dense and the effect of promoting drug absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Gel Phase Transition Process of Fatty Acid Sustained Release Composition
[0031] According to the prescription in Table 1, 200 mg of different types of fatty acids, 100 mg of progesterone, 700 mg of NMP, and 50 mg of biodegradable polymer were weighed respectively, and heated and stirred for 2 hours to obtain a clear and transparent solution.
[0032] The resulting solution was poured into 5 mL of PBS (pH=7.4), and the formability of the gel in vitro was examined. The results are shown in Table 1.
[0033] Table 1 Preparation and Formability of Different Matrix Fats
[0034] prescription Types of fatty acids mixed matrix gel time Prescription 1 stearic acid none 20s Prescription 2 Arachidic acid none 22s Prescription 3 palmitic acid none 23s Prescription 4 Lauric acid none 21s Prescription 5 myristic acid none 22s Prescription 6 behenic acid none 25s Prescription 7 palmitoleic acid no...
Embodiment 2
[0037] Prescription Screening of Different Kinds of Solvents
[0038] Weigh 20% (w / w) of fatty acid and 10% (w / w) of progesterone respectively, weigh different kinds of solvents according to Table 2, heat and stir for 3 hours, and obtain a clear and transparent solution.
[0039] The obtained solution was poured into 5 mL of PBS (pH=7.4), and the formability of the gel in vitro was examined. The results are shown in Table 2.
[0040] Table 2 Prescription screening and formability investigation of different solvents
[0041] prescription solvent Solution Appearance Formability Prescription 1 70%NMP Clarity and transparency Completely formed Prescription 2 70% PEG-400 Clarity and transparency Completely formed Prescription 3 70%PEG-600 Clarity and transparency Completely formed Prescription 4 70% ethyl lactate Clarity and transparency Completely formed Prescription 5 70% Glyceryl Triacetate Clarity and transpare...
Embodiment 3
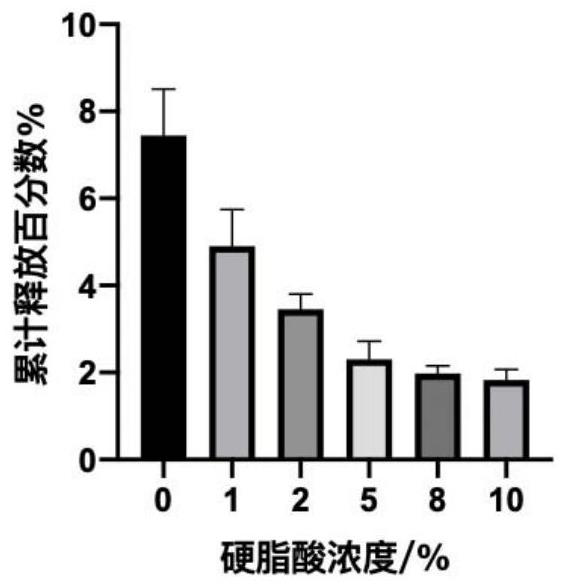
[0044] Study on the Inhibitory Burst Release Characteristics of Fatty Acids with Different Concentrations
[0045] The sustained-release composition containing 10% (w / w) of progesterone and different concentrations of fatty acids and PLGA mixed matrix injections containing 0.5% SDS-10mL PBS buffer solution (pH=7.4), in a constant temperature shaker (37 ° C, 100rmp) in vitro release test. Take out 10 mL of buffer solution at the set time point, and add an equal volume of fresh PBS buffer solution, and calculate the cumulative release rate of progesterone. The results are shown in Table 3.
[0046] Table 3 Sustained-release characteristics of different concentrations of fatty acids
[0047] prescription Fatty acid concentration (w / w) PLGA concentration (w / w) 1 hour burst Comparative example 1 0% 20% 7.79% Prescription 1 1% 19% 5.01% Prescription 2 2% 18% 3.55% Prescription 3 5% 15% 2.74% Prescription 4 8% 12% 1.95% P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com