Multifunctional nano-carrier with drug resistance and hypoxia/glutathione dual responsiveness and preparation method and application thereof
A technology of nanocarriers and drug resistance, applied in the field of multifunctional nanocarriers and its preparation, can solve problems such as poor water solubility, lack of tumor targeting, poor stability, etc., and achieve good dispersion, good biocompatibility, The effect of uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
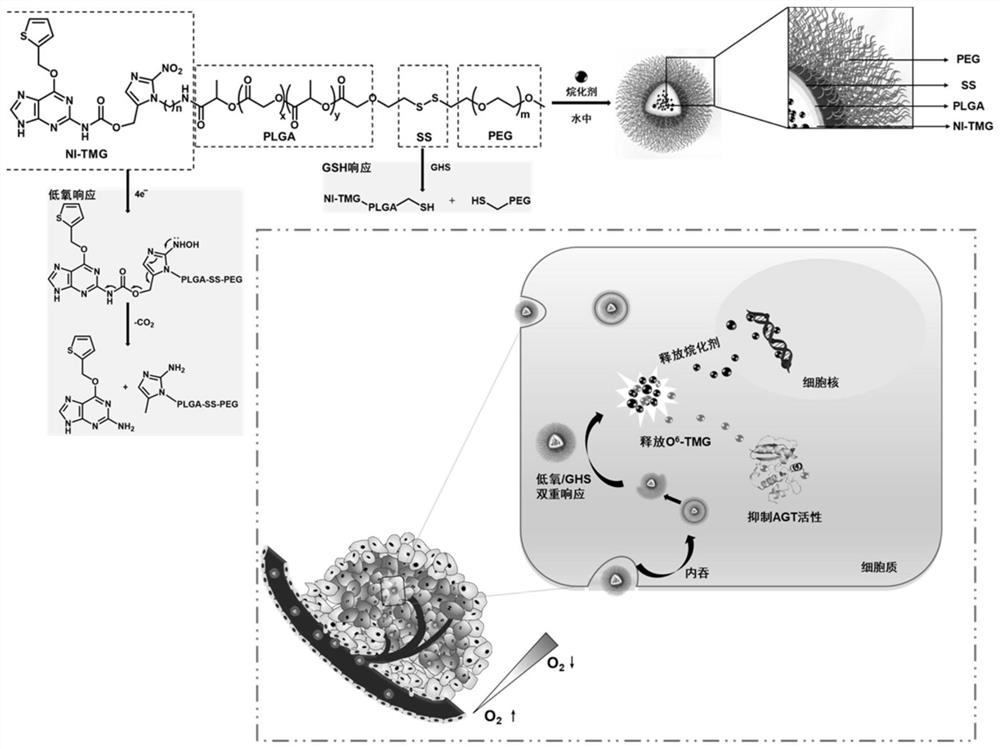
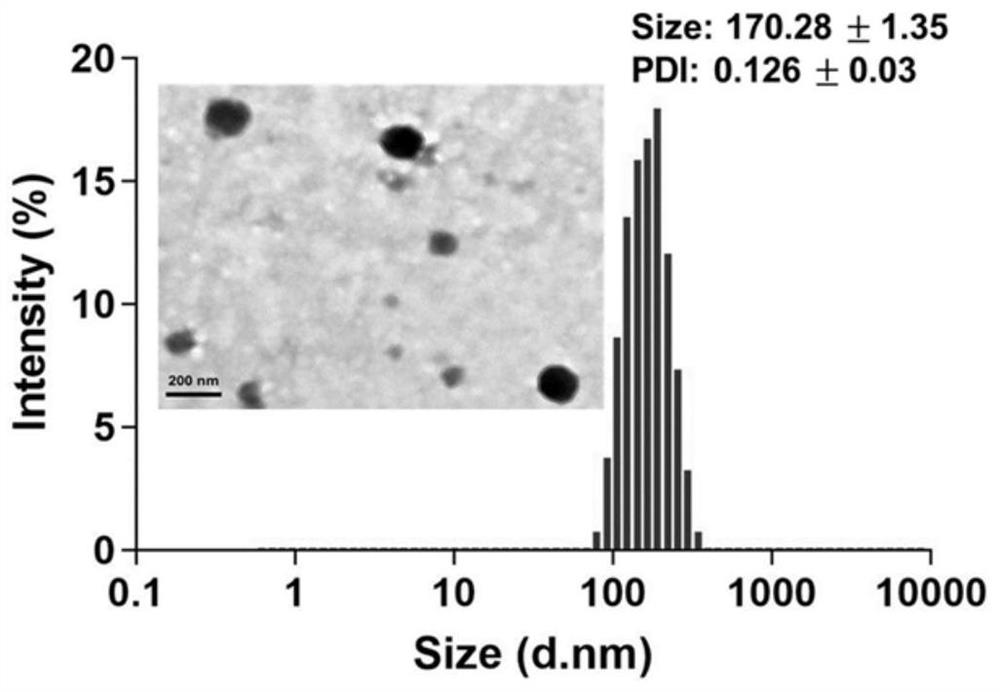
[0041] Example 1 Preparation of a multifunctional nanocarrier (NI-TMG-PLGA-SS-PEG NPs) with dual responses to drug resistance and hypoxia / GSH:
[0042] (1) Synthesis of NI-TMG-PLGA-SS-PEG
[0043] Weigh 0.12g (0.5mmol) O 6 -TMG was dissolved in 10 mL of dichloromethane to give a concentration of 12 mg / mL of O 6 -TMG dichloromethane solution, then weighed 0.15g (0.5mmol) triphosgene, under the condition of 0-10 ℃, the O 6 -TMG solution was added dropwise to triphosgene, and after the addition was completed, the 2 Under the conditions of protection and 0.25mL (3mmol) pyridine as a catalyst, react at 25°C for 4h. Then add 0.17g (1 mmol) (1-(aminoethyl)-2-nitro-1H-imidazol-5-yl)methanol, and continue the reaction at 25°C for 6h. After removing the solvent by rotary evaporation under reduced pressure at 35°C, the above reaction solution was separated and purified by column chromatography. The stationary phase was silica gel, and the mobile phase was petroleum ether and ethyl ac...
Embodiment 2
[0049] Example 2 Preparation of a multifunctional nanocarrier (NI-TMG-PLGA-SS-PEG NPs) with dual responses to drug resistance and hypoxia / GSH:
[0050] (1) Synthesis of NI-TMG-PLGA-SS-PEG
[0051] Weigh 0.2g (0.8mmol) O 6 -TMG was dissolved in 15 mL of dichloromethane to give a concentration of 13 mg / mL O 6 -TMG dichloromethane solution, then weighed 0.3g (1mmol) triphosgene, under the condition of 0-10 ℃, O 6 -TMG solution was added dropwise to triphosgene, and after the addition was completed, the 2 Under the conditions of protection and 0.72mL (5.6mmol) triethylamine as a catalyst, react at 25°C for 6h. Add 0.3g (1.8 mmol) (1-(aminoethyl)-2-nitro-1H-imidazol-5-yl)methanol, and continue the reaction at 25°C for 8h. After removing the solvent by rotary evaporation under reduced pressure at 35°C, the above reaction solution was separated and purified by column chromatography. The stationary phase was silica gel, and the mobile phase was petroleum ether and ethyl acetate. ...
Embodiment 3
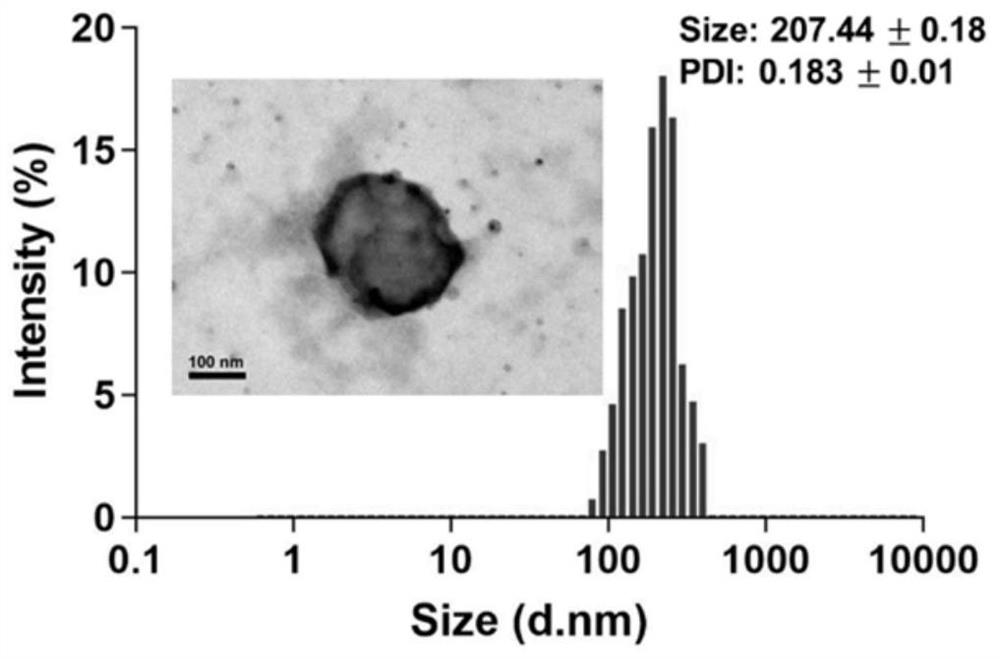
[0057] Example 3 Preparation of a multifunctional nanocarrier (NI-TMG-PLGA-SS-PEG / BCNU NPs) loaded with carmustine that has both anti-drug resistance and hypoxia / GSH dual responses
[0058] (1) Synthesis of NI-TMG-PLGA-SS-PEG
[0059] Weigh 0.25g (1mmol) O 6 -TMG was dissolved in 18 mL of dichloromethane to give a concentration of 13 mg / mL O 6 -TMG dichloromethane solution, then weighed 0.45g (1.5mmol) triphosgene, under the condition of 0-10 ℃, the O 6 -TMG solution was added dropwise to triphosgene, and after the addition was completed, the 2 Under the conditions of protection and 0.7mL (8 mmol) pyridine as a catalyst, react at 25°C for 8h. Then 0.43 g (2.5 mmol) (1-(aminoethyl)-2-nitro-1H-imidazol-5-yl)methanol was added, and the reaction was continued at 25° C. for 6 h. After removing the solvent by rotary evaporation under reduced pressure at 35°C, the above reaction solution was separated and purified by column chromatography. The stationary phase was silica gel, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com