Method for synthesizing polyhexamethylene biguanide from monoguanidine intermediate
A technology for polyhexamethylene biguanide and an intermediate is applied in the field of polybiguanide compound synthesis, can solve the problems of difficult control of the reaction process, difficult polycondensation reaction, low content of biguanide groups, etc., and achieves short production cycle and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
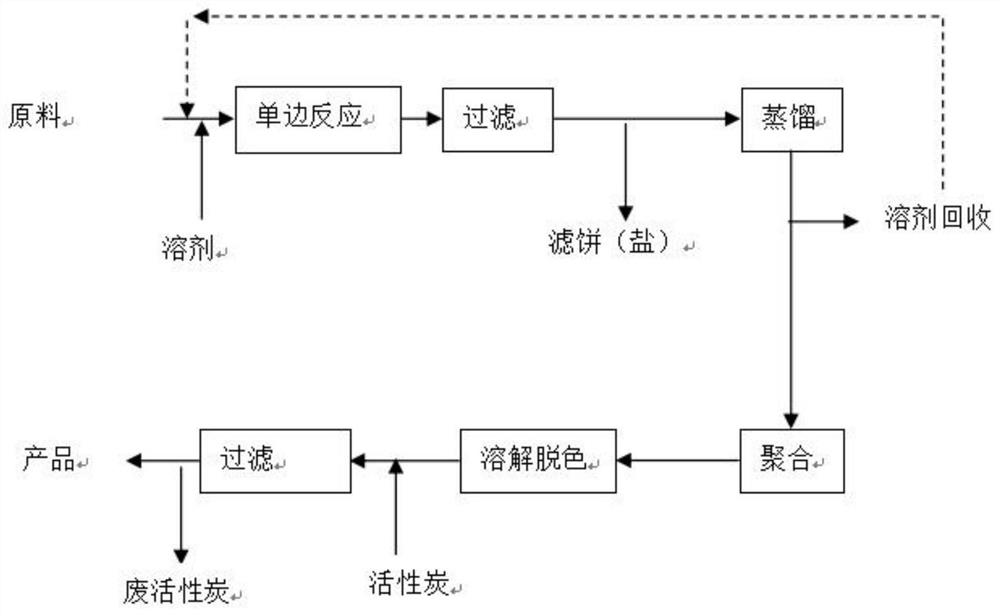
[0042] A method for synthesizing polyhexamethylene biguanide by a monoguanidine intermediate, the steps are as follows:
[0043] (1) Monomer synthesis: Add hexamethylenediamine hydrochloride (18.9g, 0.1mol), methanol 150g, sodium dicyandiamide (8.9g, 0.1mol) into the reaction kettle, heat up to reflux, stir for 2h, filter , to obtain the filtrate containing monoguanidine intermediate 1-(6-aminohexyl)-3-cyanoguanidine, the filter cake is rinsed 2 times with methanol, each with methanol 20g, and the rinse solution is combined with the filtrate;
[0044] (2) Polymerization reaction: Heating the combined filtrate in step (1), steaming out methanol (recovery), remaining viscous jelly (~22g) in the kettle, adding appropriate amount of water to aid dissolution, continuing to heat up to 140°C, stirring Polymerization reaction 4h, obtain polyhexamethylene biguanide crude product;
[0045] (3) Purification: Add 80 g of deionized water to the crude product of polyhexamethylene biguanide...
Embodiment 2
[0048] A method for synthesizing polyhexamethylene biguanide by a monoguanidine intermediate, the steps are as follows:
[0049] (1) Synthesis of monomers: add hexamethylenediamine (11.6g, 0.1mol) and 150g of n-butanol in the reaction kettle, dropwise add 22.8g of 32% hydrochloric acid, stir and heat up, and azeotropically distill off water until the temperature of the kettle is 112°C. Add sodium dicyandiamide (8.9g, 0.1mol) to the kettle again, heat up to reflux, stir and react for 2h, filter to obtain the filtrate containing monoguanidine intermediate 1-(6-aminohexyl)-3-cyanoguanidine , the filter cake is rinsed twice with n-butanol, each with 20 g of n-butanol, and the rinse solution is combined with the filtrate;
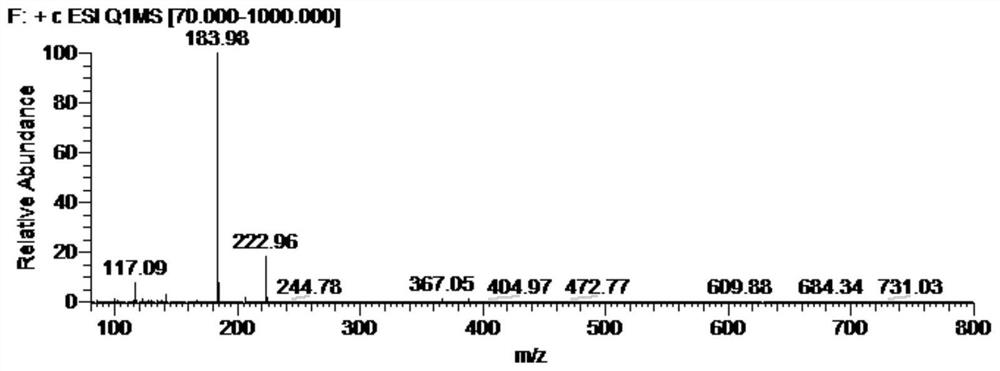
[0050] (2) Polymerization reaction: Heating the combined filtrate in step (1), after steaming out n-butanol, the remaining viscous jelly (~22g, target ion C 8 h 18 N 5 + , The theoretical molecular weight is 184.16, the test value is 183.98, and the positive...
Embodiment 3
[0054] A method for synthesizing polyhexamethylene biguanide by a monoguanidine intermediate, the steps are as follows:
[0055](1) Monomer synthesis: add hexamethylenediamine (11.6g, 0.1mol) and 150g ethanol to the reaction kettle, add dropwise 23.1g of 85% phosphoric acid, stir and heat up to reflux (~80°C), then add bis Sodium cyanamide (8.9g, 0.1mol), warming up to reflux, stirred for 4h, filtered to obtain the filtrate containing the monoguanidine intermediate 1-(6-aminohexyl)-3-cyanoguanidine, the filter cake was rinsed with ethanol Wash twice, each time with 20 g of ethanol, and combine the eluent and the filtrate;
[0056] (2) Polymerization reaction: Heating the combined filtrate in step (1), steaming out the ethanol, leaving a viscous jelly (~25g) in the kettle, adding an appropriate amount of water to help dissolve, continue to heat up to 155°C, and stir for 3h , to obtain the crude product of polyhexamethylene biguanide;
[0057] (3) Purification: Add 80 g of dei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com