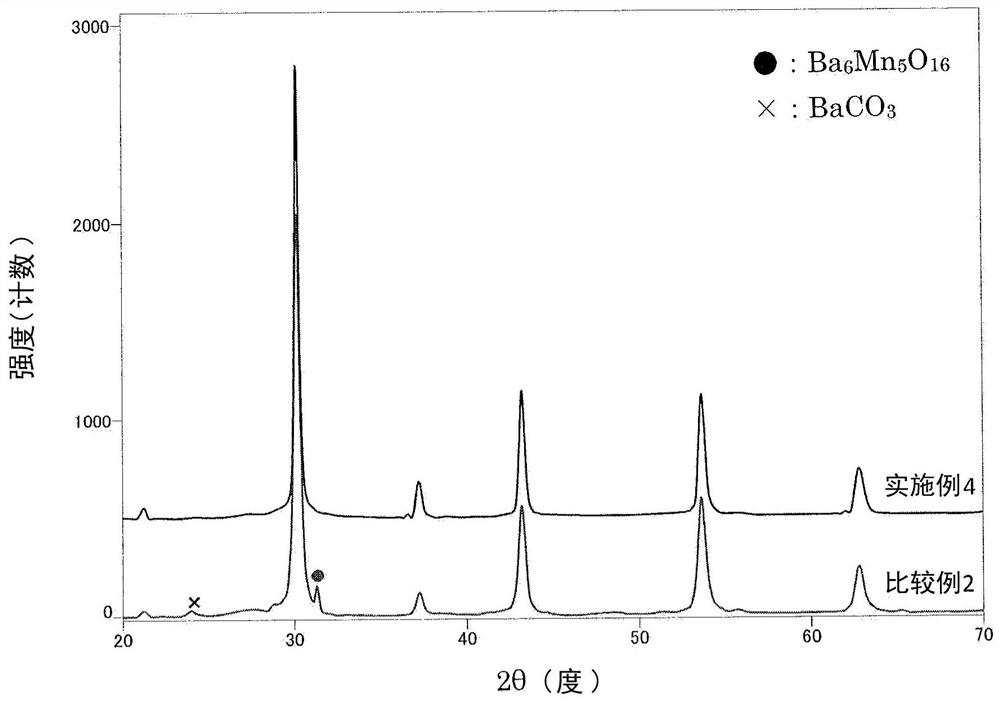
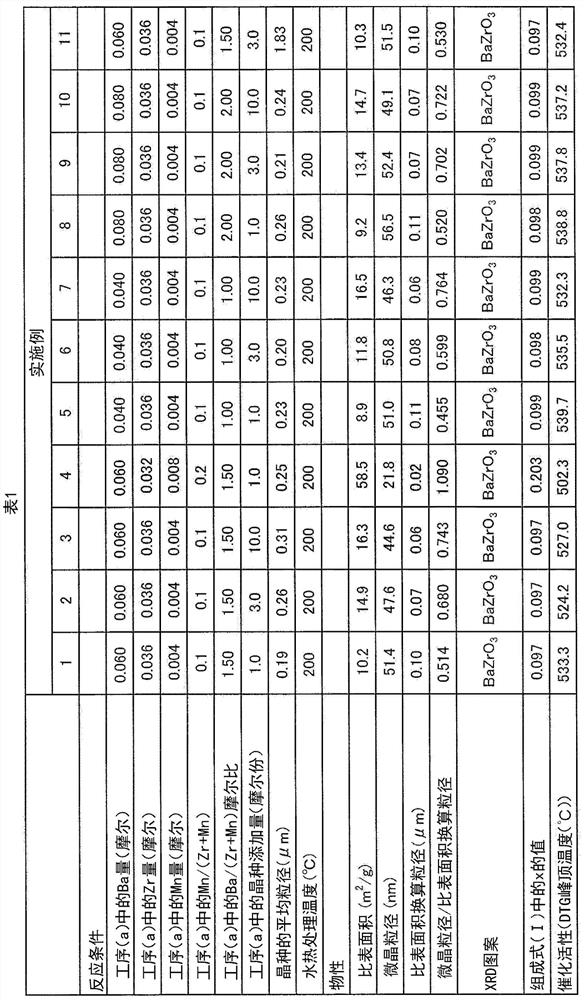
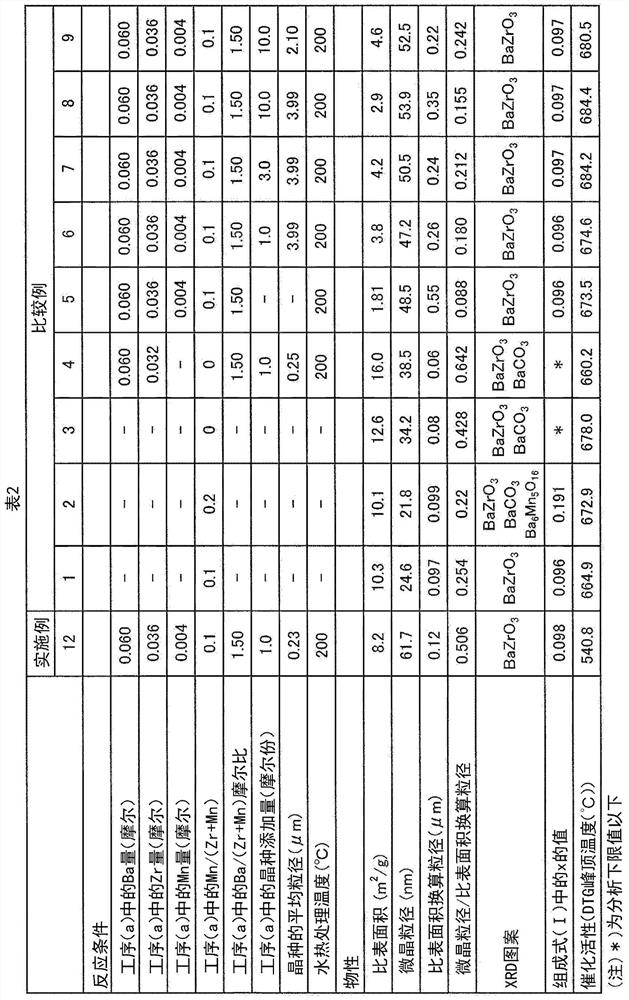
Manganese-added barium zirconate particles
A technology of barium zirconate and particles is applied in the field of barium zirconate particles added with manganese, which can solve the problems of low crystallinity and low activity, and achieve the effect of high crystallinity and high specific surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0067] (Manufacture of wet filter cake of mixed hydroxide of zirconium and manganese)
[0068] In a glass beaker, 295.29 g of zirconium oxychloride 8 hydrate (manufactured by Yoneyama Pharmaceutical Co., Ltd.) and 18.62 g of manganese chloride 4 hydrate (manufactured by Fujifilm Wako Pure Chemical Industries, Ltd.) were added to 2 L of ion-exchanged water, Stirring was performed to dissolve zirconium oxychloride octahydrate and manganese chloride tetrahydrate in water to obtain a mixed aqueous solution of zirconium salt and manganese salt. Next, 79.98 g of sodium hydroxide (manufactured by Fujifilm Wako Pure Chemical Industries, Ltd.) and 4 L of ion-exchanged water were added to a nylon beaker, stirred and dissolved to obtain an aqueous sodium hydroxide solution.
[0069] In a separate beaker equipped with a stirrer to which 1 L of ion-exchanged water was added, the above-mentioned mixed aqueous solution was added at a rate of 30 mL / min using a tube pump, and added to the abov...
Embodiment 1
[0078] (Process (a))
[0079] Weigh 51.25 g of the wet cake of the mixed hydroxide of zirconium and manganese obtained in Production Example 1 above and 18.92 g of barium hydroxide octahydrate (manufactured by Fujifilm Wako Pure Chemical Industries, Ltd.) into a titanium container, and add ion Exchange 0.09L of water, and further add 10mL of the seed crystal crushing slurry obtained in the above-mentioned Production Example 1 (0.00040 moles in terms of seeds, 1 mole part relative to 100 mole parts of the total mole parts of zirconium and manganese in the first slurry) parts), stirred to make the first slurry.
[0080] (Process (b))
[0081] The aforementioned titanium container was placed in an autoclave, heated at 200°C for 2 hours, and a mixed hydroxide of zirconium and manganese was hydrothermally reacted with barium hydroxide in the presence of the pulverized seed crystal.
[0082] (Process (c))
[0083] The slurry obtained in this way was transferred to a polyethylene ...
Embodiment 2
[0086] 0.33 g (0.0012 mol) of the seed particles obtained in Production Example 1 above was weighed into a plastic container with a capacity of 100 mL, and 8 mL of ion-exchanged water was added to prepare a slurry. After adding 8 mL of zirconia beads with a diameter of 1.0 mm to this slurry, it was installed in a planetary ball mill (P-5 manufactured by Fritsch Co., Ltd.) and operated at a rotation speed of 210 rpm for 1 hour to wet-grind the slurry. The beads were separated from the slurry using a sieve, the beads were washed with 2 mL of ion-exchanged water, and the washing water was returned to the slurry so that the total volume of the slurry was 10 mL. In this way, a pulverized slurry of seed crystals having an average particle diameter of 0.26 μm and a slurry concentration of 0.033 g / mL (0.00012 mol / mL) was obtained.
[0087] 10 mL of pulverized slurry using the above-mentioned seeds (0.0012 moles as seed crystals, 3 mole parts relative to 100 mole parts of the total mol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com