Chlorzoxazone synthesis method
A synthesis method and technology of chlorzoxazone, applied in the field of drug synthesis, can solve the problems of enhanced nucleophilicity, high cost, influence on product purity, etc., and achieve the effects of high yield and purity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
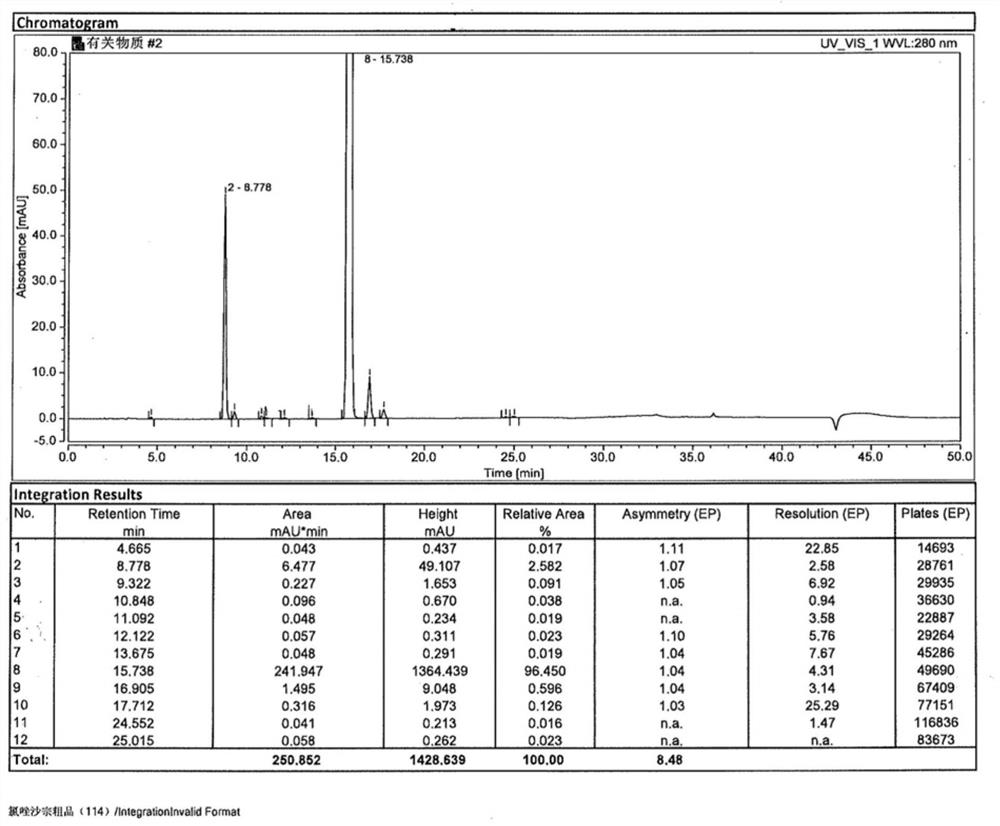
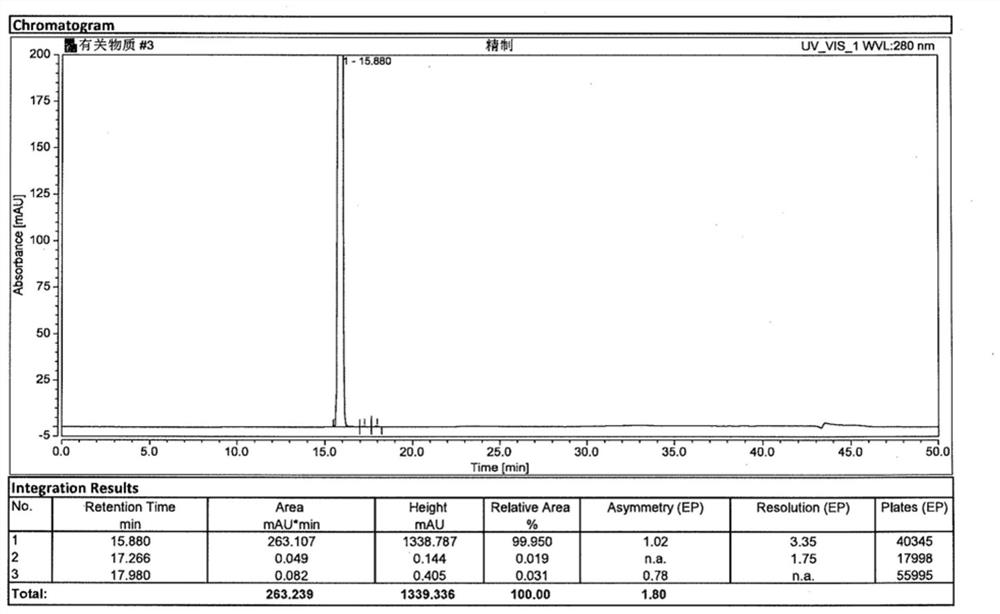
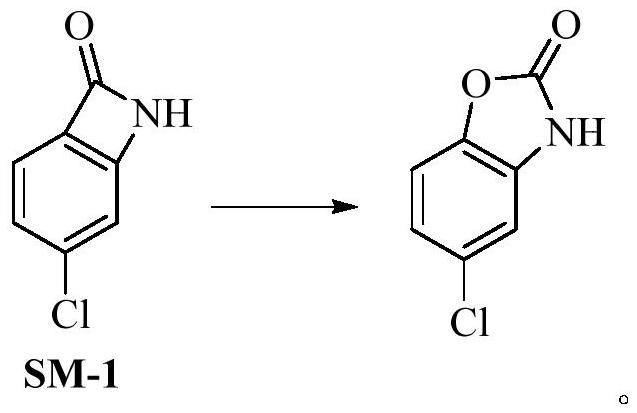
[0041] At room temperature, SM-1 (15.36g, 0.10mol), m-chloroperoxybenzoic acid (24.16g, 0.14mol), potassium bicarbonate (10.02g, 0.20mol) were added to dichloromethane (400ml), and the temperature was controlled for 10 ~15 ℃ to after the reaction finishes, add mass fraction in the reaction solution and be 10% sodium hydroxide solution (400ml), after stirring for 0.5h, separate liquid to get water phase, add mass fraction again in the organic phase and be 10% sodium hydroxide solution (250ml ), after stirring for 0.5h, separate the liquids to get the water phase, combine the water layers, adjust the pH value to 6~7 with 2mol / L hydrochloric acid, filter to obtain the crude product, and the gained crude product is subjected to ethanol / purified water (V 乙醇 :V 纯化水 =1:3, 240ml) system was recrystallized to obtain chlorzoxazone with a total yield of 93.4% and a purity of 99.950%.
Embodiment 2
[0043] At room temperature, add SM-1 (15.36g, 0.10mol), m-chloroperoxybenzoic acid (18.12g, 0.105mol), sodium carbonate (21.20g, 0.20mol) into dichloromethane (400ml), and control the temperature for 20~ 25 ℃ to after the end of the reaction, add mass fraction in the reaction solution and be 10% sodium hydroxide solution (400ml), after stirring for 0.5h, separate liquid to get the water phase, add mass fraction again in the organic phase and be 10% sodium hydroxide solution (250ml) , after stirring for 0.5h, separate the liquids to get the water phase, combine the water layers, adjust the pH value to 6-7 with 2mol / L hydrochloric acid, filter to obtain the crude product, and obtain the crude product through ethanol / purified water (V 乙醇 :V 纯化水 =1:3, 240ml) system was recrystallized to obtain chlorzoxazone with a total yield of 91.3% and a purity of 99.870%.
Embodiment 3
[0045] At room temperature, add SM-1 (15.36g, 0.10mol), m-chloroperoxybenzoic acid (17.26g, 0.10mol), potassium carbonate (27.64g, 0.20mol) into dichloromethane (400ml), and control the temperature for 25- 30 ℃ to after the end of the reaction, adding mass fraction in the reaction solution is 10% sodium hydroxide solution (400ml), after stirring for 0.5h, separate the liquids to get the water phase, then add mass fraction in the organic phase and be 10% sodium hydroxide solution (250ml) , after stirring for 0.5h, separate the liquids to get the water phase, combine the water layers, adjust the pH value to 6-7 with 2mol / L hydrochloric acid, filter to obtain the crude product, and obtain the crude product through ethanol / purified water (V 乙醇 :V 纯化水 =1:3, 240ml) system recrystallized to obtain chlorzoxazone with a total yield of 89.2% and a purity of 99.865%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com