Drug screening method and three-dimensional tumor slice model culture method
A screening method and three-dimensional culture technology, applied in the field of biomedicine, can solve the problems of inability to obtain cell or tissue information at the molecular level, time-consuming, unfavorable treatment and recovery of patients, and achieve the effect of accelerating precise anti-cancer treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
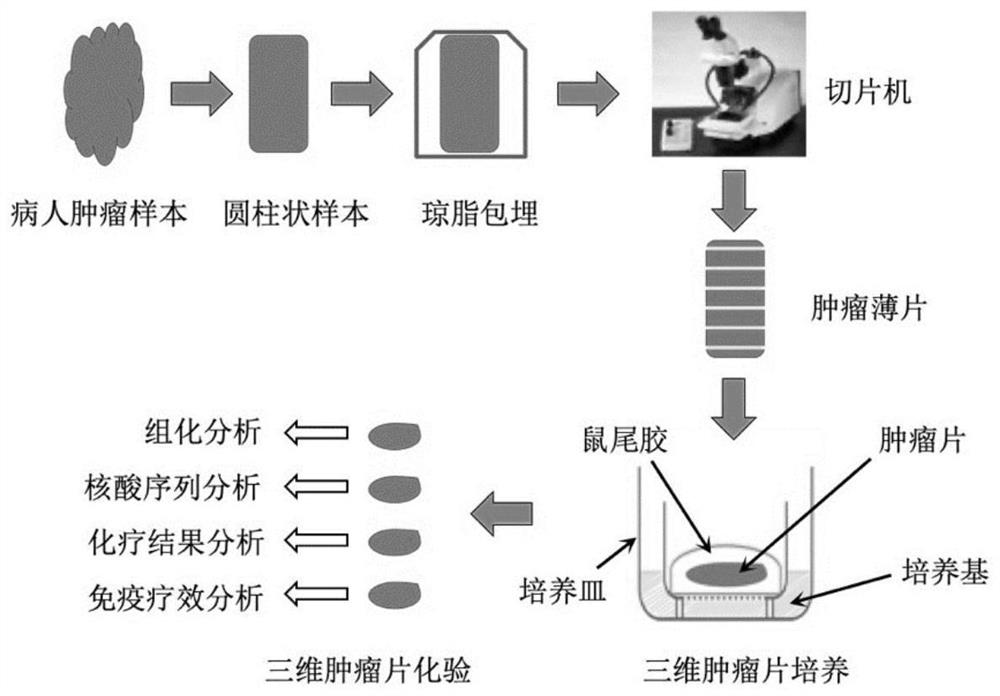
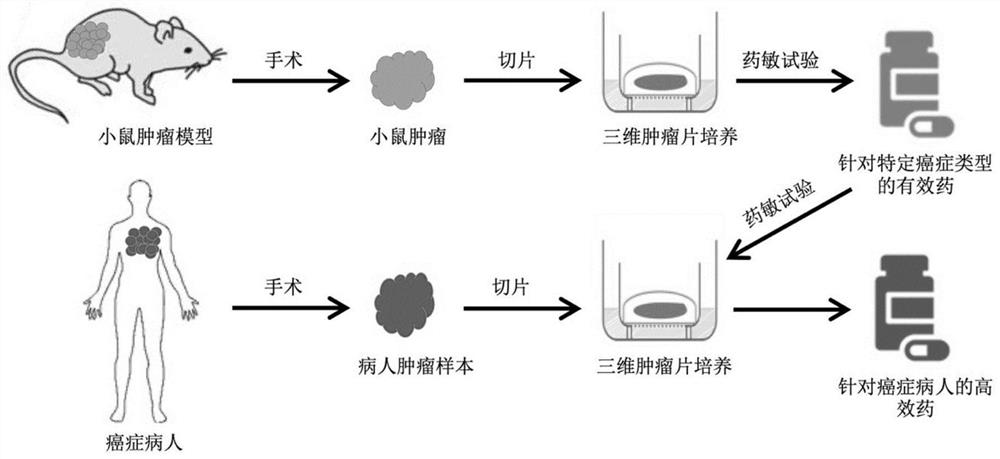
[0028] An embodiment of the present invention provides a drug screening method, which includes the following steps: performing three-dimensional culture on the three-dimensional tumor slice model; wherein, the preparation method of the three-dimensional tumor slice model includes: the tumor labeled with the apoptosis reporter substance The tissue is sliced to obtain the three-dimensional tumor slice model; then, the three-dimensional tumor slice model in three-dimensional culture is processed with candidate drugs, and the candidate drugs are screened based on the signal of the apoptosis reporter substance, which can be referred to figure 1 .
[0029] In this paper, "treating the three-dimensional tumor slice model in three-dimensional culture with a candidate drug" may refer to: contacting the drug with the three-dimensional tumor slice model, specifically by adding the drug to the three-dimensional culture medium. The three-dimensional tumor slice model established by the i...
Embodiment 1
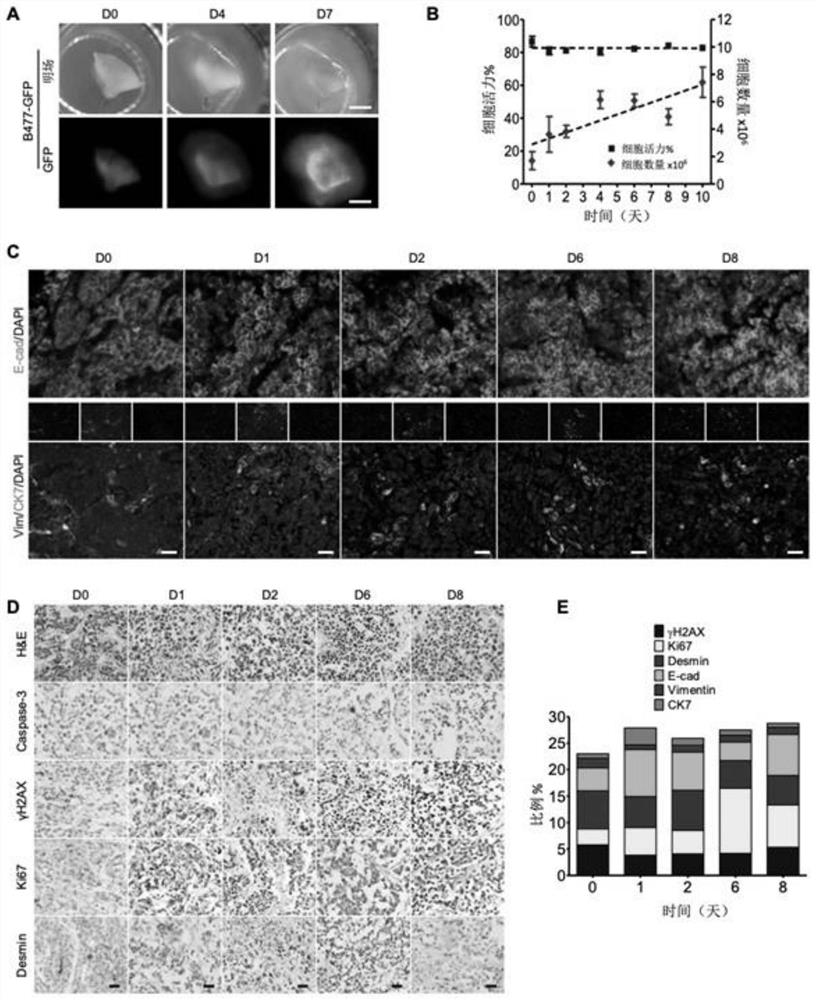
[0059] Three-dimensional tumor slice models are able to maintain the cellular repertoire and immune components of their original tumors.
[0060] Three-dimensional tumor slice models were prepared as follows: Fresh tumors were embedded in low-melting point agarose and sliced at a thickness of 300 μm with a vibrating microtome. Then, tumor slices were placed on an air-liquid interface system for culture, and time-course images of the slices were captured from the original tumor before culture (referred to as day 0, D0) to D 7 in culture, using a Leica M165FC fluorescence stereomicroscope .
[0061] Among them, the culture conditions of three-dimensional tumor slices are as follows: A (rat tail collagen I), B (10X Ham's F-12) and C (sterile buffer, 2.2g NaHCO 3 In 100ml0.05N NaOH and 200mM HEPES) according to the ratio of 8:1:1 preparation. Take the prepared 100 μL gel solution and spread it on the membrane (membrane pore size 0.4mm) in the Millicell insert cell culture inne...
Embodiment 2
[0072] Based on FRET time-lapse technology and MTT endpoint method to predict the efficacy of chemical drugs in the three-dimensional tumor slice platform.
[0073] Due to the lack of fluorescent markers in clinical cancer samples, this example uses GFP-labeled cells, C3-labeled cells and non-fluorescent-labeled cells in three-dimensional tumor slices to test the drug response. First, cell viability of fluorescent samples was measured in cisplatin-treated B477-GFP breast tumor sections ( Figure 6 Middle A-B). An increase in GFP signal was found within 7 days during the three-dimensional tumor slice, and this increase was prevented after cisplatin treatment, such as tumor slice size, GFP intensity, etc. These phenomena were consistent with the cell viability determined by the MTT assay.
[0074] MDA-MB-231-C3 tumor slices were next treated with 1 μM bortezomib (drug), and drug responses were measured in a time series study. found that the FRET ratio decreased in a stepwise f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com