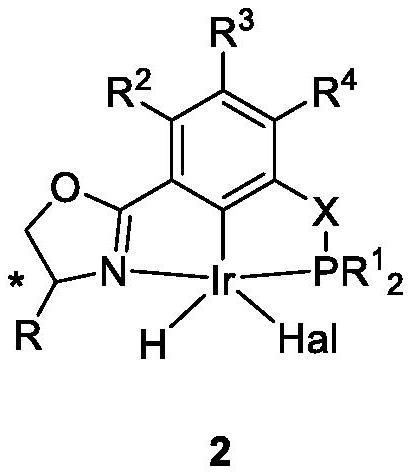
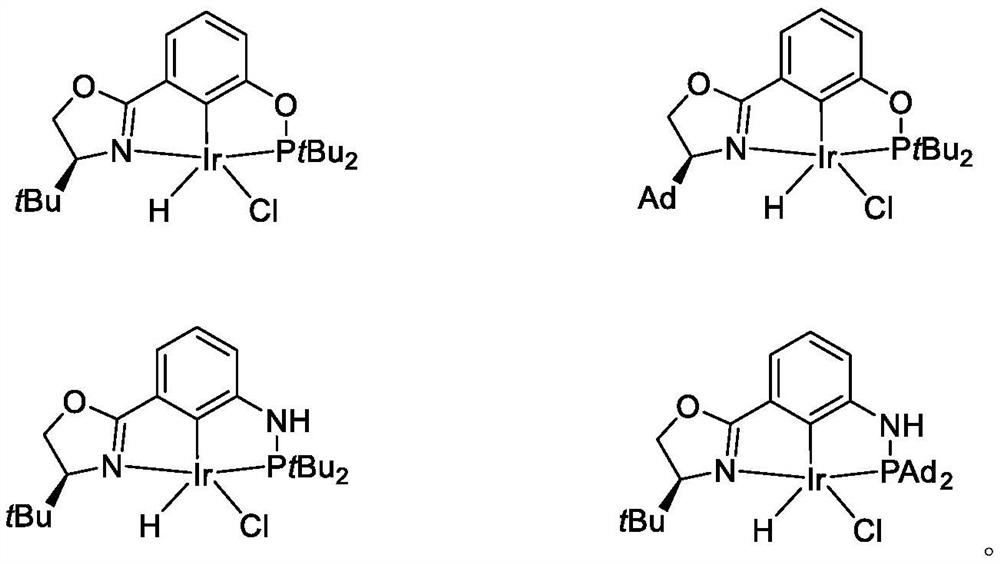
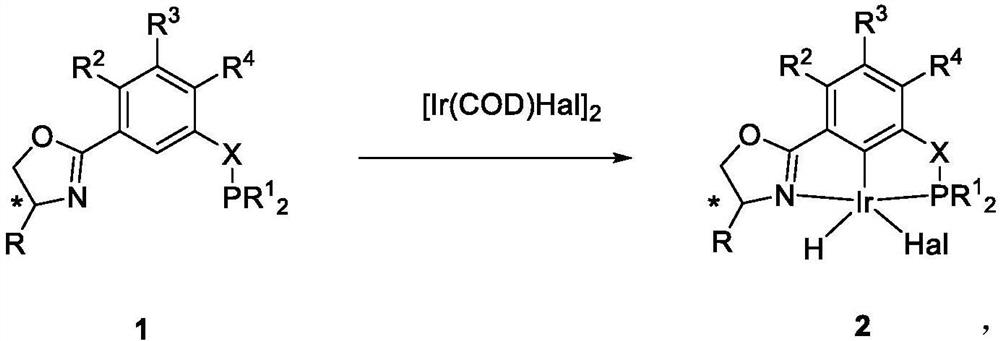
NCP ligand, metal iridium complex thereof, and preparation method and application of complex
A compound and an independent technology, applied in the field of NCP ligands, can solve problems such as lack, and achieve good regioselectivity, good enantioselectivity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0154] Examples 1-4: Preparation of Ligands 1A-1D
[0155] Preparation of Ligand 1A: (S)- tBu NC O P tBu
[0156] step 1:
[0157]
[0158] Add 3-cyanophenol (1.9 g, 10 mmol), ZnCl 2 (2.7g, 20mmol), anhydrous chlorobenzene (60mL), L-tert-leucinol (2.1g, 18mmol), and the reaction system was heated to reflux for 24h. The system was cooled to room temperature, ethyl acetate (20 mL) and water (30 mL) were added to dissolve the solids in the system, filtered, the filtrate was separated, and the organic phase was washed with saturated NaHCO 3 solution (3×10mL) and saturated NaCl (20mL), washed with anhydrous NaCl 2 SO 4 It was dried, concentrated by filtration, and purified by column chromatography (ethyl acetate:petroleum ether=1:4) to obtain a white solid 4a (1.01 g, 46%), with a purity of >97% by HS.
[0159] 1 H NMR (400MHz, CDCl 3 )δ9.07(s,1H),7.54(s,1H),7.36(d,J=7.7Hz,1H),7.19(t,J=7.9Hz,1H),6.92(d,J=8.1Hz, 1H), 4.35(dt, J=15.6, 9.2Hz, 2H), 4.08(dd, J=10.0, 6.9Hz,...
Embodiment 5~8
[0186] Embodiment 5~8: preparation complex 2A~2D
[0187] Preparation of complex 2A:
[0188]
[0189] In an argon glove box, ligand 1A (0.5mmol, 181.5mg) and [Ir(COD)Cl] 2 (0.24mmol, 161.0mg) was added to a Schleck sealed tube equipped with a stirring bar, and then 10mL of toluene solvent was added, the sealed tube was sealed, and the glove box was taken out. The reaction was reacted in the bath for 12 hours, then the reaction system was cooled to room temperature, the solvent was sucked dry under high vacuum, the residue was washed with n-pentane (3×30 mL) and filtered through a fritted funnel equipped with diatomaceous earth, the filtrate was collected, and then The solvent was pumped dry under high vacuum condition to obtain the red complex 2A (162.5 mg, 55%), with a purity of >97% in H spectrum.
[0190] 1 H NMR (400MHz,C 6 D. 6 )δ7.21(d, J=7.5Hz, 1H), 6.80(d, J=7.7Hz, 1H), 6.72(t, J=7.7Hz, 1H), 4.29(dd, J=9.4, 3.5Hz, 1H), 4.20(dd, J=9.1, 3.5Hz, 1H), 3.63(t, J=9....
Embodiment 9
[0203] Example 9: Catalytic activity experiment of complex 2A on the asymmetric transfer hydrogenation reaction of 1,1-diaryl substituted ethylene
[0204] Take the transfer hydrogenation process of 1-(2-methyl)phenyl-1-phenylethene 8a as an example:
[0205]
[0206] First in the argon gas glove box, the complex 2A (0.004mmol), NaO t Bu (0.006mmol), EtOH (5.43mmol, 0.25mL), 1-(2-methyl)phenyl-1-phenylethene 8a (48.5mg, 0.25mmol) were added to a 10mL sealed tube. After the reaction was stirred at room temperature for 36 h, it was quenched by exposure to air. Then the solvent was removed by rotary evaporation, and flash column chromatography (a mixture of petroleum ether and ethyl acetate as eluent, petroleum ether:ethyl acetate=40:1) gave colorless liquid 9a.
[0207]
[0208] (S)-1-Methyl-2-(1-phenylethyl)benzene (9a). Colorless liquid (48.0mg, 98%), purity>97% by H-spectrum. 1 H NMR (400MHz, CDCl 3 )δ7.18(m,4H),4.31(q,J=7.1Hz,0H),2.22(s,1H),1.60(d,J=7.2Hz,1H). 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com