Application of piceatannol in preparation of medicine for preventing and/or treating herpes simplex virus infection
A technology of herpes simplex virus and piceatanol, which is applied in the field of medicine, achieves good market application prospects and improves the effect of physical condition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Piceatannol effect in vitro inhibition of herpes simplex virus: Example 1
[0032] 1. Experimental Methods
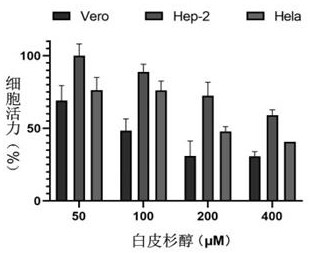
[0033] 1.1 Cytotoxicity test of piceatannol
[0034] Determination piceatannol cytotoxicity in three cell lines, the Vero, Hela, Hep-2 cells were seeded in 96-well plates, after 24 h, disposable suction original culture medium, serial dilutions of the piceatannol ( final concentration of 400 μM, 200 μM, 100 μM, 50 μM) was added a 96 well plate, three wells for each concentration, while with the control group. Cells were placed in 37 ℃, containing 5% CO 2 After the temperature in the cell culture incubator 24h, aspirated the drug, 4% paraformaldehyde fixed at room temperature 15 min, aspirated, 5% crystal violet staining rt 15 min, measured using a microplate reader A dry after washing 540nm value.
[0035] A cell viability test group = 540nm / A negative control group 540nm × 100%.
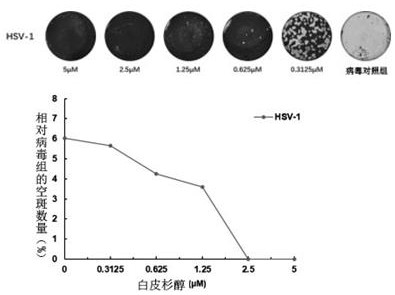
[0036] 1.2 cytopathic effect (CPE) inhibition test piceatannol detection of the viru...
Embodiment 2
[0053] Example 2: Mechanism piceatannol inhibition of HSV virus
[0054] 1. Experimental Methods
[0055] 1.1 plaque forming assay
[0056] Vero cells were seeded in 12-well plates to its covered with a monolayer. 1.2 following the experimental procedure in four different modes of administration Wedelia lactone were added (final concentration 10 μM), 24h after the supernatant was collected, while the virus is provided in the control group. The supernatant was diluted 10 charges -1 ~ 10 -3 Times, 37 [deg.] C cells adsorbed to 1 h, aspirated, the prepared overlay medium was added, After cooling solidification, inverted at 37 ℃ incubator. After the spots to which fixing stained for plaque counting.
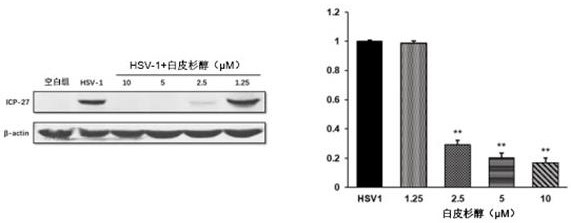
[0057] 1.2 Western blot test
[0058] Vero cells were seeded in 6-well plates, cells covered monolayer, serial dilutions piceatannol after HSV-1 and HSV-2 in the pre-mixed 37 ℃ 1 h, added to 6-well plates, 37 ℃ 1 H, aspirated, and replaced with maintenance medium, and with the virus cont...
Embodiment 3
[0072] Example 3: Effect of piceatannol treat HSV infected mice
[0073] 1. Experimental Methods
[0074] 1.1 Experimental modeling
[0075]All mice used in this case are in line with the humanitarian treatment of the Chinese science and technology experiment (VGKFCZ-2006-398). Babl / C female mice using three weeks of age, weight 10 to 12 g, randomly divided into normal control group, virus control group, positive drug acyclovir group (10 mg / kg / day), white psol High dosing group (5 mg / kg / day), white pine fedol low dose group (2.5 mg / kg / day), a total of 5 groups. The mice were infected by dripping nose, and 50 μl of viruses were added to the nasal cavity of each mouse. The normal control group dropped with the amount of physiological saline.
[0076] 1.2 Administration method and clinical observation
[0077] After 4 h after viral infection, it was administered in the abdominal injection, and acyclovir was administered at 10 mg / kg / day dose, white skin fiol was 5 mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com