Near-infrared molecular probe based on malondialdehyde response, preparation method and application thereof
A molecular probe and near-infrared technology, applied in the field of biosensors, can solve the problems of insufficient wavelength of MDA probes, probes limited to cell imaging, weakened effectiveness, etc., to improve short wavelength, poor penetration depth, and preparation speed Fast, achieve the effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
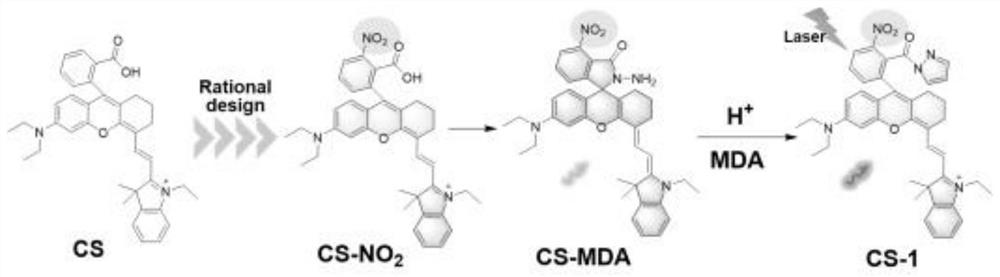
[0062] The preparation of embodiment 1 CS-MDA molecular probe
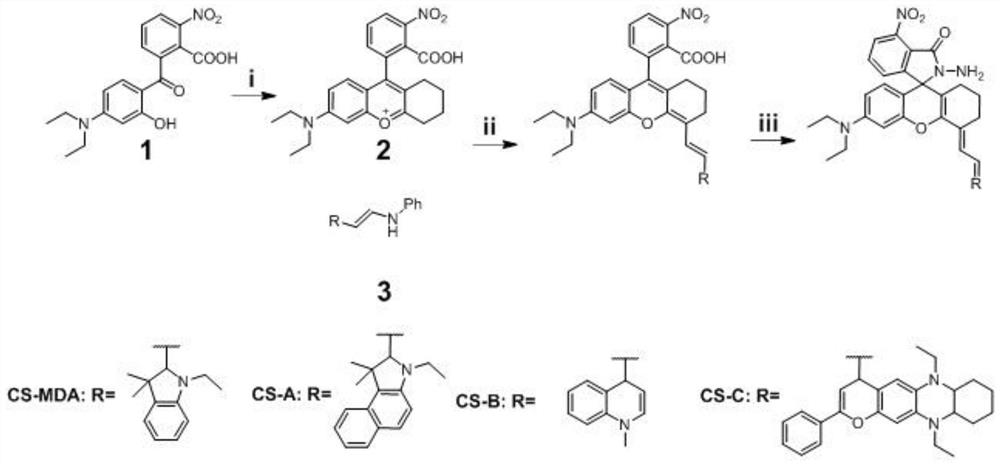
[0063] like figure 2 Cyclohexanone (1 mL) was added dropwise to concentrated H 2 SO 4 (10 mL), then, 1 g of compound 1 (2-(4-(diethylamino)-2-hydroxybenzoyl)-6-nitrobenzoic acid) was added under stirring, the reaction mixture was stirred vigorously, and Heated at 90°C for 2 hours, then poured into ice water, and then the precipitated precipitate was filtered and washed quickly with water and petroleum ether to finally obtain compound 2(9-(2-carboxy-3-nitrophenyl)-6-( Diethylamino)-1,2,3,4-tetrahydroxanthine), a dark red viscous liquid, can be used in the next step without further purification;
[0064] Compound 2 and compound 3 ((E)-N-(2-(1-ethyl-3,3-dimethylindol-2-yl)vinyl)aniline) were dissolved in AcOH containing AcOH 2 In O, the molar ratio of compound 2 and compound 3 was 1:1, and then the reaction mixture was heated to 55 ° C for 60 minutes, and then the reaction system was extracted with saturated bri...
Embodiment 2
[0066] Example 2 Preparation of CS-A Molecular Probe
[0067] like figure 2 As shown, the synthesis method is the same as in Example 1, except that the compound 3 added is (E)-N-(2-(1-ethyl-3,3-dimethylbenzindol-2-yl)vinyl) aniline.
Embodiment 3
[0068] Example 3 Preparation of CS-B Molecular Probe
[0069] like figure 2 As shown, the synthesis method is the same as in Example 1, except that the added compound 3 is (E)-1-methyl-4-(2-(phenylamino)vinyl)quinoline-1-dimethyl.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com