System for preparing sodium carbonate and baking soda as byproducts boric acid and ferric hydroxide from trona liquid
A technology for ferric hydroxide and by-product boric acid, which is applied in the directions of ferric oxide/ferric hydroxide, boron oxides, boron compounds, etc., can solve the problems of product quality impact, increase of iron content, decrease of high-quality product rate, etc. The effect of superiority and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without creative efforts fall within the protection scope of the present invention.
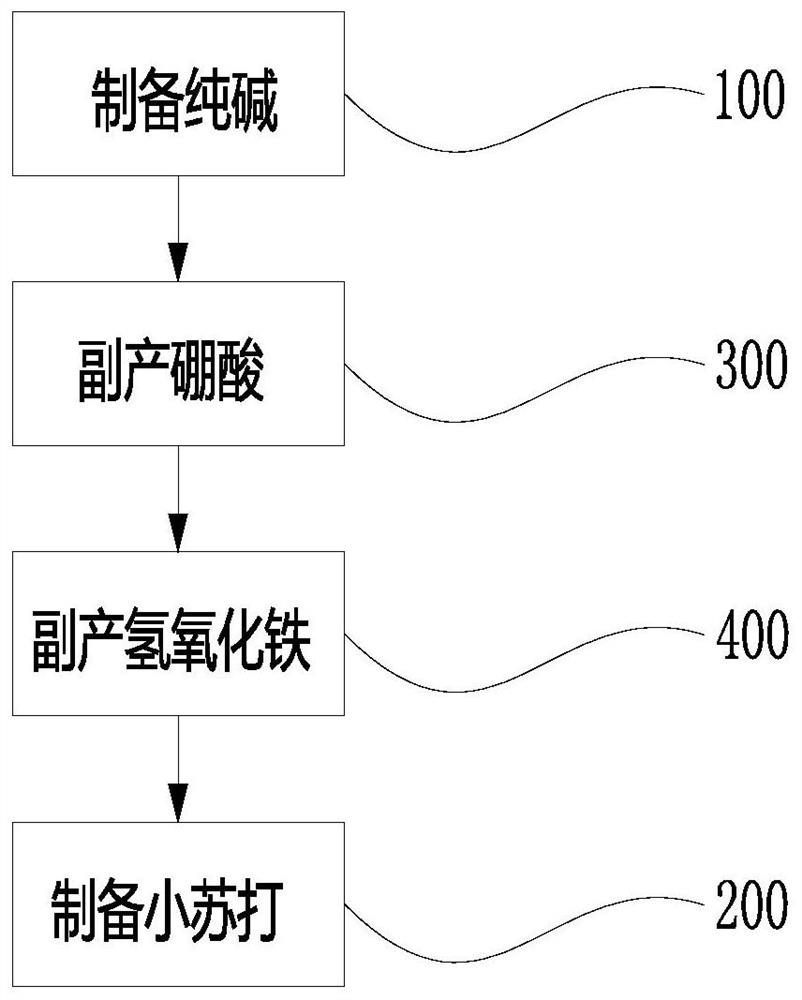
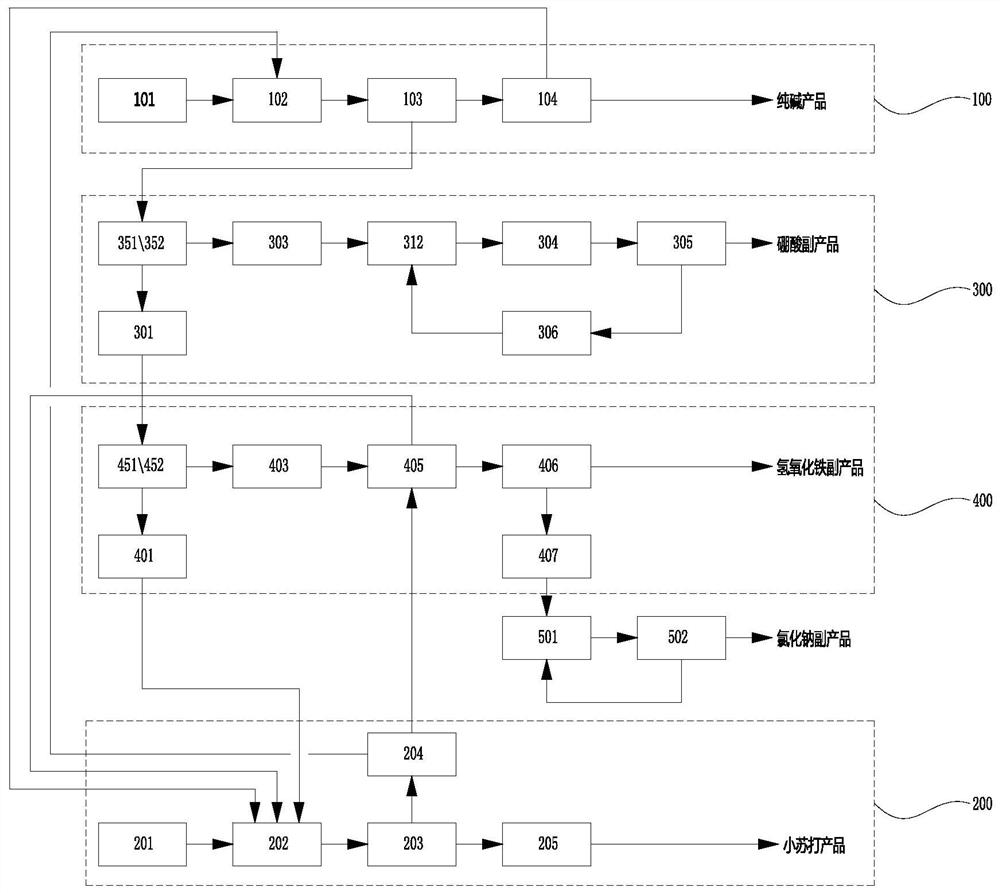
[0031] like Figure 1 to Figure 4 The system of a kind of natural lye system soda ash and baking soda byproduct boric acid and ferric hydroxide described, it comprises soda ash preparation unit 100, boric acid byproduct unit 300, ferric hydroxide byproduct unit 400 and sodium bicarbonate preparation unit 200,
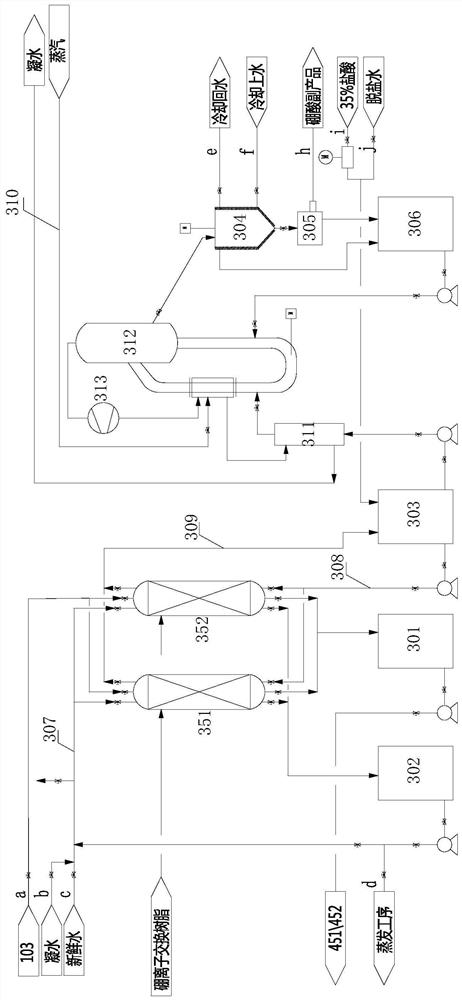
[0032] Soda ash preparation unit 100 comprises natural lye pipe 101, soda ash evaporation crystallizer 102, soda ash filtration separation device 103 and ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com