Catalytic method for methane dry reforming
A methane dry reforming, methane technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of limited conversion rate and product selectivity of methane and carbon dioxide , side reaction products and other problems, to achieve excellent thermal stability, improve selectivity, improve selectivity and yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of described nickel-based boron nitride catalyst comprises the steps:
[0032] 1) putting boron nitride into isopropanol for ultrasonication, and then drying it to obtain a thin boron nitride carrier;
[0033] 2) mixing the metallic nickel salt solution with the dried flake boron nitride, and then drying it to obtain the dried mixture;
[0034] 3) Calcining the dried mixture in air to obtain the nickel-based boron nitride catalyst.
[0035] In step 1), the mixing method is to put boron nitride in the isopropanol solution; the mass volume ratio of the boron nitride to the isopropanol solution is 1: (50-1000), and the ultrasonic time is 12h-36h,
[0036] In step 2), the mixing method is to drop the metal nickel salt solution onto the boron nitride carrier; the mass ratio of nickel nitrate and boron nitride carrier in the metal nickel salt solution is (0.002-0.04): 1 , the drying temperature is 80°C-110°C, and the drying time is 8-24h;
[0037] I...
Embodiment 1
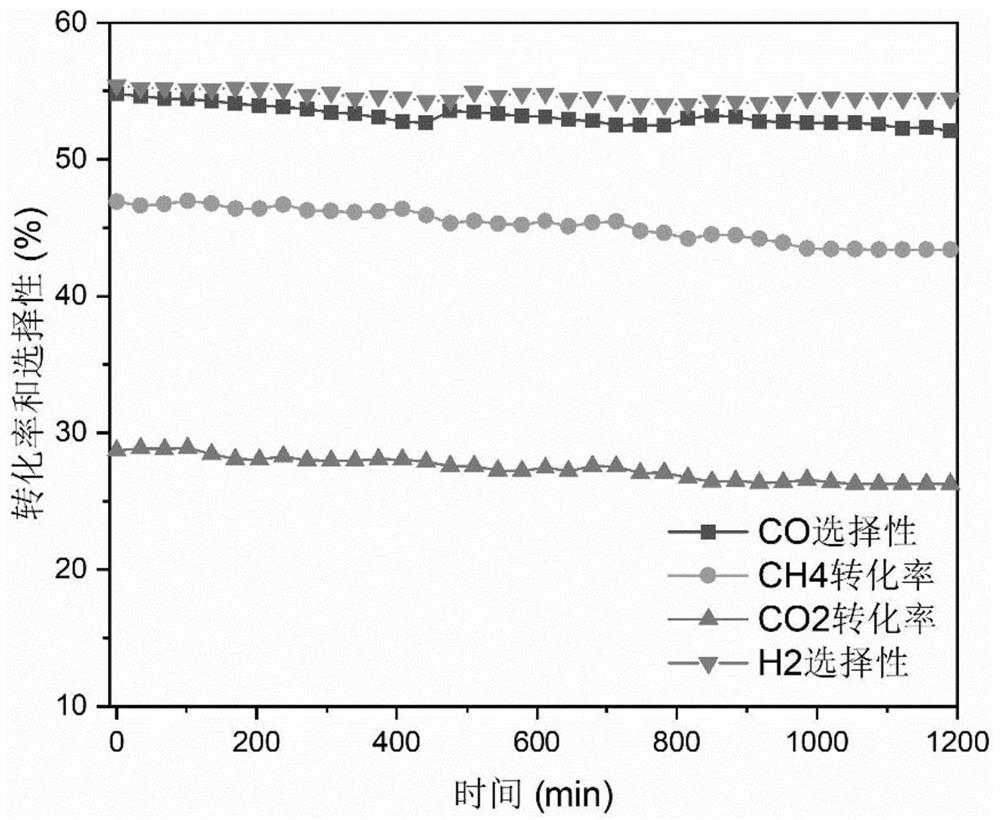
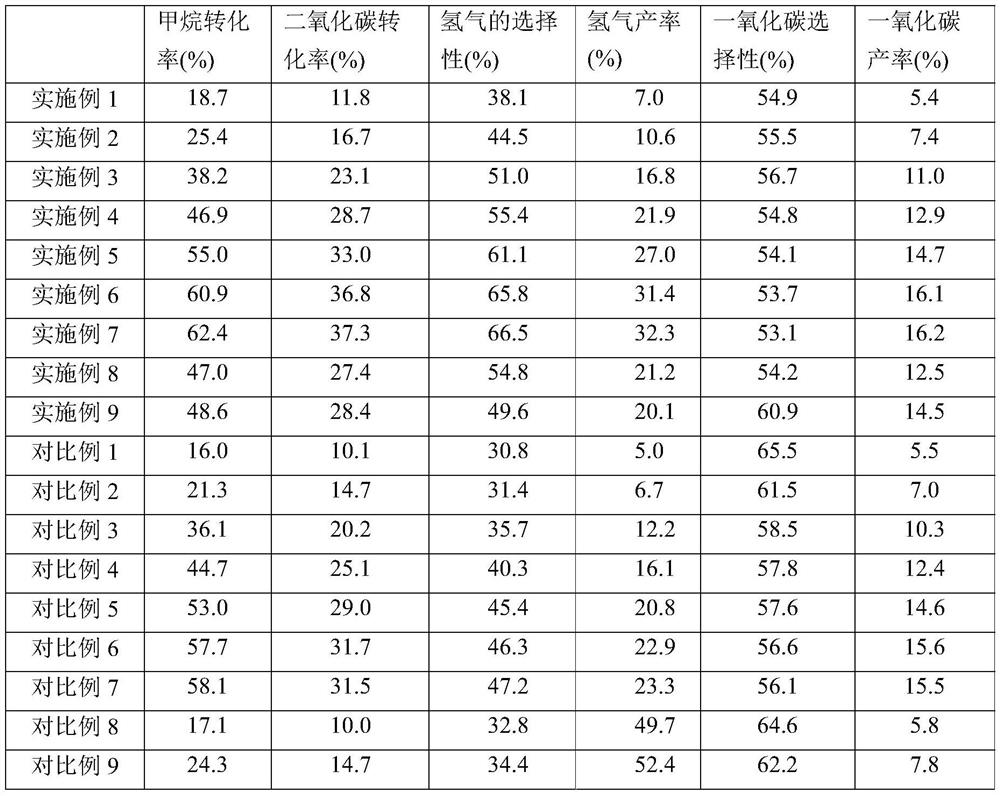
[0040] Mix methane, carbon dioxide and argon to obtain a mixed raw material gas (the volume ratio of methane, carbon dioxide and argon in the mixed raw material gas is 1:1:1), and mix the mixed raw material gas for 720h -1The volume space velocity passes into DBD low-temperature plasma reactor (the discharge area of described DBD low-temperature plasma reactor is equipped with 1g nickel-based boron nitride catalyst, and the active component of described nickel-base boron nitride catalyst is metallic nickel, The carrier is boron nitride, and the metal nickel in the nickel-based boron nitride catalyst is 4% by mass in the catalyst) for catalytic reaction, the reaction temperature is 25°C, the reaction pressure is normal pressure, and the mixing The feed gas passes through the dielectric barrier discharge region at a flow rate of 60ml / min. The power of the DBD low-temperature plasma reactor is 21.8w, and the discharge frequency is 7.4kHz to prepare the product synthesis gas (hyd...
Embodiment 2
[0048] Mix methane, carbon dioxide and argon to obtain a mixed raw material gas (the volume ratio of methane, carbon dioxide and argon in the mixed raw material gas is 1:1:1), and mix the mixed raw material gas for 720h -1 The volume space velocity passes into DBD low-temperature plasma reactor (the discharge area of described DBD low-temperature plasma reactor is equipped with 1g nickel-based boron nitride catalyst, and the active component of described nickel-base boron nitride catalyst is metallic nickel, The carrier is boron nitride, and the nickel-based boron nitride catalyst (heavy metal nickel in the catalyst is 4% by mass) carries out a catalytic reaction, the reaction temperature is 35° C., and the reaction pressure is 0.1 MPa. The reaction raw material The residence time of gas in the dielectric barrier discharge area is 8.5s, the power of the DBD low-temperature plasma reactor is 29.4w, the discharge frequency is 7.4kHz, and the prepared catalytic products synthesi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com