Platycodon grandiflorum almond paste and preparation method thereof
A technology of almond and platycodon, which is applied in the direction of ointment delivery, pharmaceutical formulations, and medical preparations of non-active ingredients. It can solve the problems of loss of smell, feeling uncomfortable, and irreversible, and achieves small toxic and side effects, uniform color, and improved health. The effect of immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The technical scheme adopted in the present invention is as follows: a platycodon grandiflora almond paste, comprising the following components in parts by weight: 120-140 parts of platycodon grandiflorum, 120-140 parts of almond, 40-50 parts of ginseng, 175-185 parts of astragalus, 40 parts of licorice -50 parts, Poria cocos 175-185 parts, Perilla 85-95 parts, Angelica dahurica 85-95 parts, Ginger 120-140 parts, Scrophulariaceae 120-140 parts and honey 700-1000 parts, make 90-145 bags.
[0030] 135 parts of bellflower, 135 parts of almond, 45 parts of ginseng, 180 parts of astragalus, 45 parts of licorice, 180 parts of poria cocos, 90 parts of perilla, 90 parts of angelica, 135 parts of ginger, 135 parts of scrophulariaceae and 880 parts of honey, made into 135 bags .
[0031] 130 parts of bellflower, 130 parts of almond, 42 parts of ginseng, 178 parts of astragalus, 42 parts of licorice, 178 parts of poria cocos, 88 parts of perilla, 88 parts of angelica, 133 parts of...
experiment example 1
[0038] Experimental Example 1: Determination of Astragaloside IV:
[0039] Preparation of reference substance solution: Take an appropriate amount of astragaloside IV reference substance, weigh it accurately, add methanol to make a solution containing 0.5mg per 1ml, and obtain it.
[0040] Preparation of the test solution: take two bags of platycodon grandiflora and almond paste, take them out, dry them in a vacuum drying oven to constant weight, grind the dry paste, take 3g of dry paste powder and weigh it accurately, put it in a Soxhlet extractor, add methanol 40ml, Soak in cold overnight, add an appropriate amount of methanol, heat and reflux for 4 hours, recover the solvent from the extract and concentrate to dryness, add 10ml of water to the residue, dissolve it with slight heat, shake and extract 4 times with n-butanol saturated with water, 40ml each time, combine the normal Wash the butanol solution twice with ammonia test solution, 40ml each time, discard the amine sol...
example 2
[0043] Example 2: Toxicological experiment data of Platycodon grandiflora and almond paste
[0044] Drugs to be tested: Platycodon grandiflorum almond extract, alanine aminotransferase reagent, blood urea nitrogen determination reagent;
[0045] Test animals: healthy ICR white mice, SD white rats, closed group.
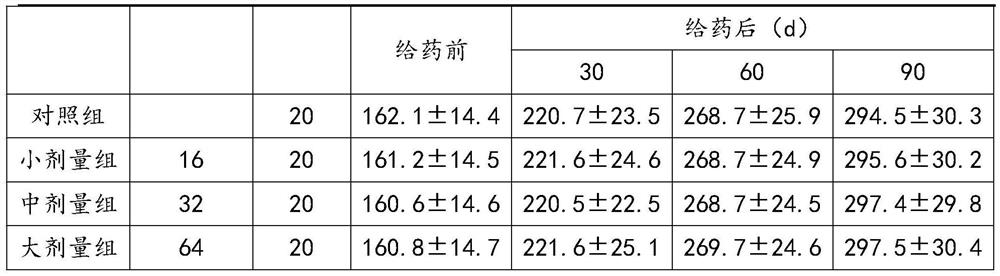
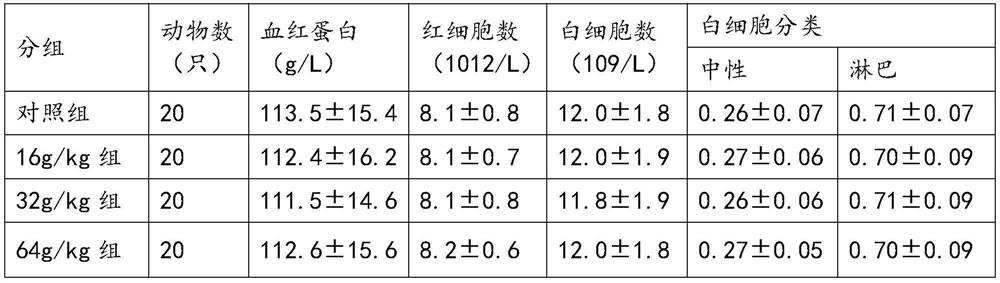
[0046] Acute toxicity test on mice by intragastric administration: 60 healthy ICR mice, weighing 18-22g, half male and half male, were randomly divided into 3 groups according to body weight and sex. After the animals fasted for 14 hours, the first and second groups Gastrointestinal 30g / kg and 60g / kg of Platycodon grandiflora and almond paste were given twice in the morning and afternoon, with an interval of 6 hours. The third group was the control group, which was given water by intragastric administration. The animals were housed in separate cages under the same conditions, with solid feed and free drinking water, and observed at room temperature for 7 days. The c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com