Preparation method of ruxolitinib phosphate
A technology of aloth phosphate and tini, which is applied in the field of medicine, can solve the problems of low chiral purity, high cost of noble metal catalysts, unsuitable for scale-up production, etc., and achieve high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of 4-chloro-7 - ((2- (trimethylsiliconyl) ethoxy) methyl) -7H-pyrrole [2,3-D] pyrimidine (Compound 2)
[0056] First, several 4-chloro-7H-pyrrols are first soluble in 3x volume N, N-diethylthycelamide, nitrogen protection, cooling - 10 ° C or less, sodium hydride is added. (1.05 eq), after the addition, the temperature is warmed to 20 to 25 ° C, stirred for 1 to 2 h, then control the temperature of -5 ° C or lower, dried 2- (trimethylsilyl) ethoxymethyl chloride (1.05 eq), after the bet is finished, the temperature is 10 to 25 ° C, the reaction is 1 to 2 h, TLC monitors to the end reaction, and 6-fold volume saturated ammonium chloride solution is added to quench the reaction. Map the precipitated solid, the filter cake was washed with water and a tertiary ether. 40 ~ 45 ° C drying, 4-chloro-7 - ((2- (trimethylsilicilicidal) ethoxy) methyl) -7H-pyrrole [2,3-D] pyrimidine, yield 95 %, Purity ≥ 94.0%. Directly used in the next reaction.
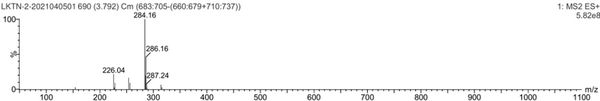
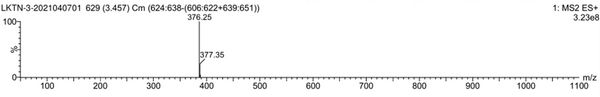
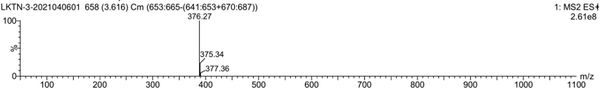
[0057] Mass spectrum figu...
Embodiment 2
[0059] Preparation of 4-chloro-7 - ((2- (trimethylsiliconyl) ethoxy) methyl) -7H-pyrrole [2,3-D] pyrimidine (Compound 2)
[0060] First, several 4-chloro-7H-pyrrols [2,3-D] pyrimidine are dissolved in 4x volume N, N-diethylcene amide, nitrogen protection, cooling - 10 ° C or lower, sodium hydride is added batch (1.05 eq), after the addition, the temperature is warmed to 20 to 25 ° C, stirred for 1 to 2 h, then control the temperature of -5 ° C or lower, dried 2- (trimethylsilyl) ethoxymethyl chloride (1.0 Eq), after the bet is finished, the temperature is 20 to 25 ° C, the reaction is 1 to 2 h, and TLC is monitored to the end reaction, and the addition of 6-fold volume saturated ammonium chloride solution is added to quench the reaction. Map the precipitated solid, the filter cake was washed with water and a tertiary ether. 40 ~ 45 ° C blast drying, 4-chloro-7 - ((2- (trimethylsilica) ethoxy) methyl) -7H-pyrrole [2,3-D] pyrimidine, yield 91 %, Purity ≥ 91.0%. Directly used in the ...
Embodiment 3
[0062] 4- (4,4,5,5-tetramethyl-1,3,2-dioxolacytic ring-2-yl) -7 - ((2- (trimethylsiliconyl) ethoxy) Base) -7H-pyrrole and [2,3-D] pyrimidine (Compound 3)
[0063] First, several 4-chloro-7 - ((2- (trimethylsilicilicidal) ethoxy) methyl) -7H-pyrrol is first soluble in 4x volume dimethyl sulfoxide , Nitrogen protection, cooling to -10 ° C or lower, dripping isopropyl magnesium chloride (1.05 eq), after completion, temperature rise to 0 ~ 5 ° C, stir the reaction 1 to 2 h, then control temperature 0 ~ 5 ° C or less, add The dry boronic acid-cell-ravate (1.05 eq), after the addition, 0 to 5 ° C for 1 to 2 h, TLC monitored to the end reaction, 6-fold volume saturated ammonium chloride solution to quench the reaction. 6x glycetate extraction, water washing, saturated brine washing, water washed, mixed with water, filtered, concentrated under reduced pressure to concentrate 4- (4, 4, 5, 5-tetramethyl-1, 3, 2) - Dioxolam-2-yl) -7 - ((2- (trimethylsilicilicide) ethoxy) methyl) -7H-pyrrol [...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com